Imperial’s COVID-19 vaccine moves to next phase
by Ryan O'Hare
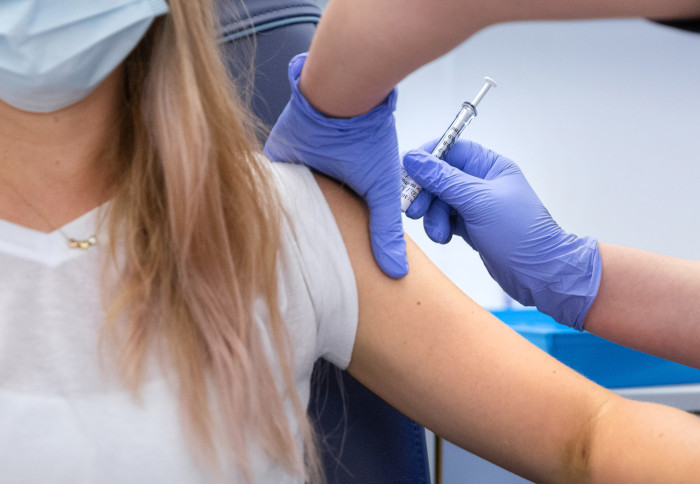
Clinical trials to test a new coronavirus vaccine developed by researchers at Imperial College London are progressing to the next phase.
Earlier this week clinical staff moved to vaccinate a larger number of participants in order to assess the optimal dosage of Imperial's COVID-19 vaccine candidate, following the successful initial phase with 15 volunteers.
In this next phase of the trial, 105 participants aged 18-75 will be randomised to receive their first shot of one of three doses of the vaccine at a west London facility, followed by a booster four weeks later. The three arms of the study are blinded, so neither participant nor clinical staff will know which dose they have received.

The first 15 volunteers, who were part of the initial dose escalation phase, will also return to receive their second booster dose in the coming days.
All participants are being closely monitored by the clinical team. In addition to recording any potential adverse reactions to the vaccine the team will analyse participants’ blood for the presence of neutralising antibodies against the SARS-CoV-2 virus.
Dr Katrina Pollock, clinical lead on the Imperial COVAC1 study, said: “We have had a promising start but it remains too early to speculate whether our vaccine candidate will be effective in preventing infection. The early clinical trials are progressing well and I would like to thank all those who are supporting this work, particularly our trial volunteers.”
Professor Robin Shattock, who is leading the development of Imperial’s COVID-19 vaccine, said: “The progression to the next phase of the trial is an important step in assessing the safety of our vaccine. Analysing blood samples for antibodies and T-cell response will provide some indication of whether our vaccine can produce an immune response to fight the virus.
"Larger clinical trials will still be needed to tell us whether our vaccine candidate, and any other COVID-19 vaccine in development, may be successful in reducing the spread or severity of COVID-19.”
Imperial's clinical trials are the first test of a new self-amplifying RNA (saRNA) technology, which has the potential to revolutionise vaccine development and enable scientists to respond more quickly to emerging diseases.
The vaccine has undergone rigorous pre-clinical safety tests and in animal studies it has been shown to be safe and produced encouraging signs of an effective immune response.
Preclinical findings published this month in Nature Communications showed that two doses of the vaccine produced highly specific neutralising antibodies against SARS-CoV-2 in mice, which were able to neutralise the virus.
The Imperial vaccine has been developed through more than £40 million from the UK government as well as philanthropic support from hundreds of donors to Imperial's COVID-19 Response Fund.
As part of the vaccine development, Imperial has formed a new social enterprise VacEquity Global Health (VGH), in partnership with Morningside Ventures, to rapidly develop vaccines and distribute them as widely as possible in the UK and overseas, including to low- and middle-income countries.
See the Imperial COVID-19 Vaccine Trial and COVID-19 Response Fund webpages for further information.
How to take part
If you a member of the public interested taking part in the trial, please check the Trial Website.
Full details for participants can be found in the sidebar 'Imperial Vaccine Trial' or visit www.imperial.ac.uk/covid-19-vaccine-trial/
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Ryan O'Hare
Communications Division
