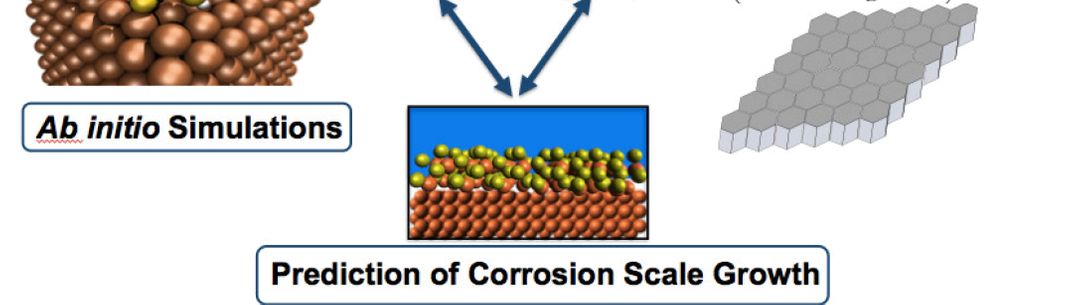
The presence of solid corrosion products, such as iron sulphides and iron carbonates, can lead to a significant reduction in corrosion rates. Therefore, a fundamental understanding at the atomistic level of their formation, stability and growth is vital for the oil and gas field equipment sustainability. Particularly, the formation of iron sulphides is complicated by the existence of several stable and metastable Fe-S compounds1,2. To date a comprehensive understanding of the phase stability has not yet been achieved3,4. Atomistic calculations combined with ab initio thermodynamics are used to characterise the structural, electronic and thermodynamic properties of the corrosion scale phases.
Furthermore, a continuum growth model for the nascent scale is developed. It is a generic model applicable to different types of materials. The growth rate is calculated taking into account explicitly the reactions at the metal-scale, and scale-environment interfaces together with the transport through the scale. Within the model time dependent drift-diffusion-reaction equations for any set of mobile species are solved. A simplified grain structure of the scale is considered and the model can be applied to various chemical environments. A C++ computer code for scale growth calculations is being implemented. For predictive simulations the growth model parameters will be calculated from ab initio calculations.
Computational Materials Science