Our research aims at enabling the large-scale electrochemical conversion of renewable energy to fuels and valuable chemicals and vice versa. Such processes will be critical in order to allow the increased uptake of renewable energy.
Energy storage or conversion is favoured by the reaction/interaction of a substrate in the electrolyte with electrode materials such as electrocatalysts or intercalation media. The processes occurring at the interface between the electrolyte and electrode of interest are therefore governing the electrochemical processes' efficiency. Our work focuses on the extensive characterisation and fundamental understanding of these interfacial processes, in electrolysers, fuel cells, and batteries setups.
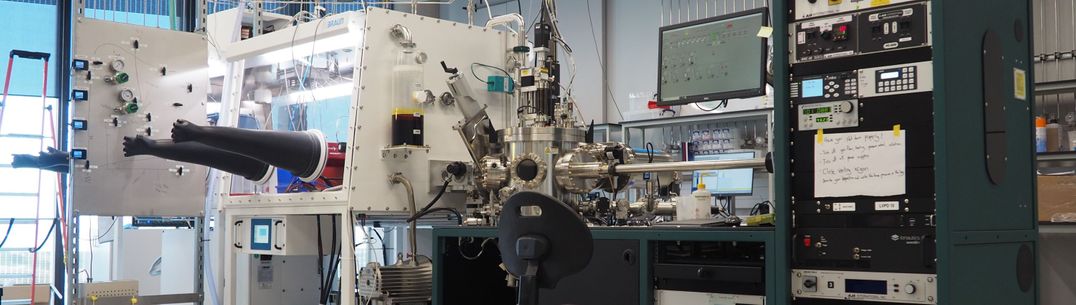
Using our high-end materials design and characterisation facilities, we hope to shed light on the key reactions driving or hindering each electrochemical process, highlight similarities and differences between them, and provide fundamental guidance on how to tailor the corresponding interface to its specific needs, toward efficient and stable electrochemical devices.
Several electrochemical applications are under investigation in our group:
- Water electrolysis for the storage of renewable electricity in the form of Hydrogen as a carbon-free fuel.
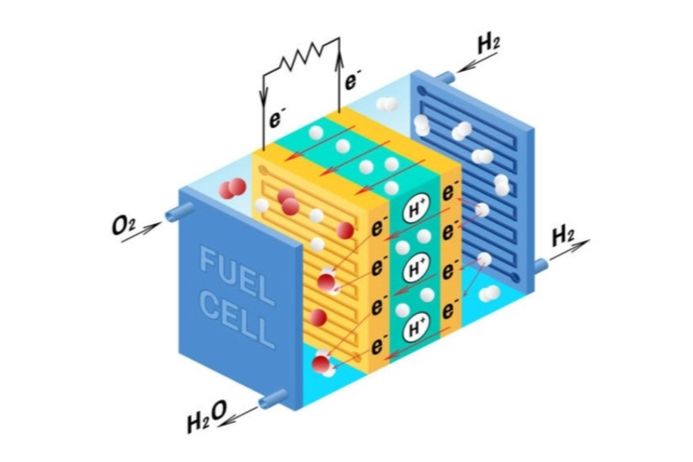
- Hydrogen Fuel Cells reversing water splitting for H2-powered devices, improving stability and availability of catalysts.
- Li-ion Batteries for renewable electricity storage towards zero-emission transport and grid storage.
- N2 Reduction to NH3 for the local production of fertilisers, shipping fuels, and N-containing industrial chemicals.
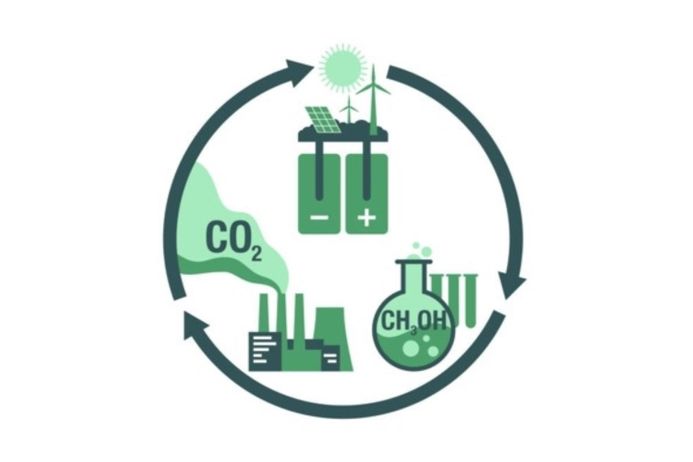
- CO2 Reduction to green fuels or value-added products.
- Biomass valuation for the synthesis of valuable chemicals, polymers, and green fuels.
See our recent publications here.