Corrosion of reinforcing steel is a major deterioration process affecting reinforced concrete structures worldwide. Corrosion causes cracking and spalling of the concrete cover, loss of load bearing capacity and ultimately structural collapse. Cracking of the cover is often a critical limit state and this can be modelled as a two-stage process consisting of an initiation phase, defined as the time taken for corrosion to commence, and a propagation phase, where the formation of corrosion products induces expansive stresses and damage. Until recently, most research has focused on the time up to corrosion initiation, while the propagation phase leading to failure remains poorly understood.
One important aspect that lacks understanding is the amount of corrosion products that must form to cause damage. It is likely that not all corrosion products contribute to cracking because some are soluble species that dissolve in the pore solution and migrate into adjacent cement paste away from the sites of corrosion. Thus, the ability of the pores to accommodate rust by acting as repositories may extend the period between onset of corrosion and stress development.
This project aims to enhance understanding of the effect of corrosion on the microstructure of reinforced concrete, in particular the nature, amount and distribution of the corrosion products, how they accumulate and cause damage.
Approach
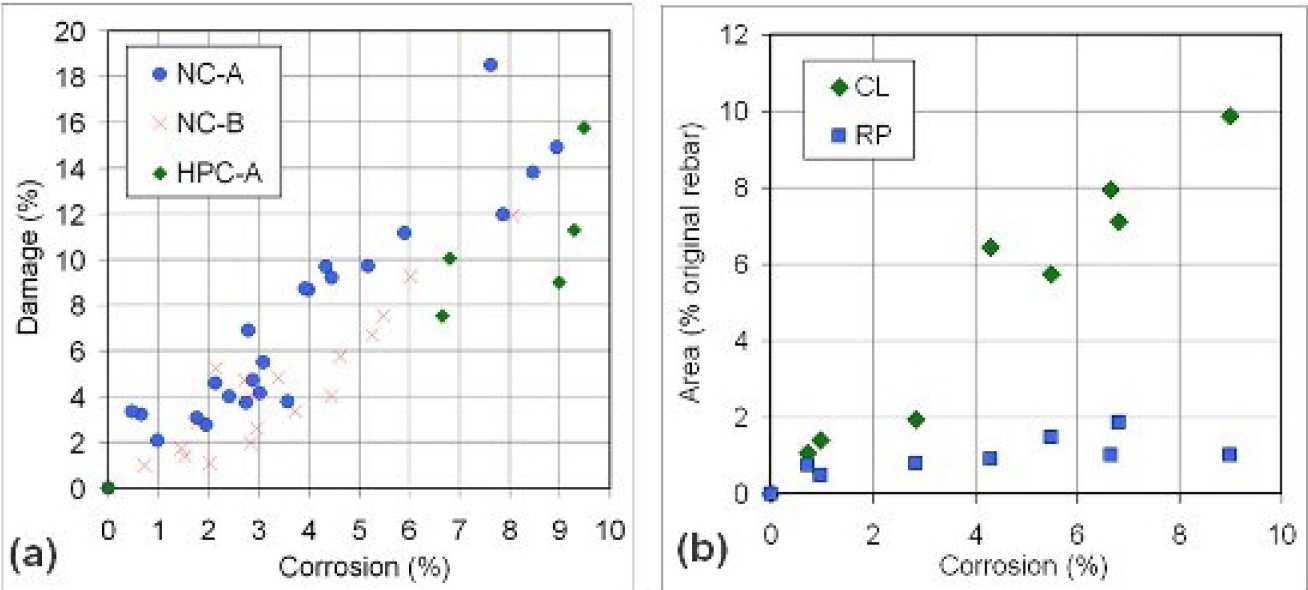
Reinforced concrete panels (Fig. 1a) were prepared with Portland cement (0.44w/c) and ternary blended cement (40% slag, 30% fly ash, 0.345w/b). The panels were cured for 14 days, then placed in an artificial environmental chamber and subjected to cyclic salt spray for 12 months to induce corrosion. Each cycle consisted of spraying with 3.53%wt NaCl solution for 4 hrs and drying at 40oC for 68 hrs. A series of 8mm-thick cross-section slices was produced from each panel. The slices were then epoxy-impregnated, polished and examined with optical microscopy, backscattered electron microscopy and energy dispersive X-ray microanalysis. Image analysis was used to measure the Fig 1: Degree of corrosion and damage amount of cracking and corrosion products (Fig 1b).
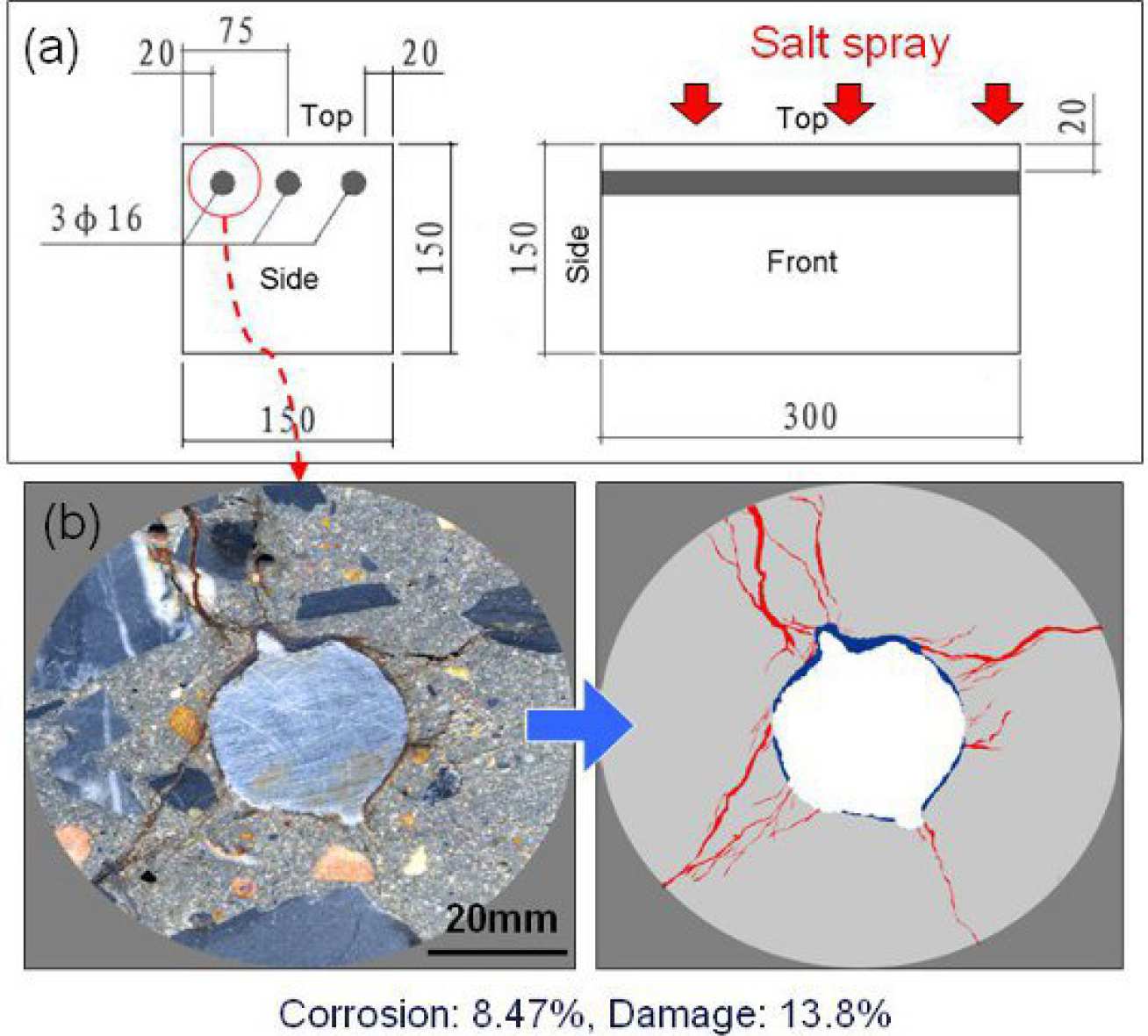
Observations
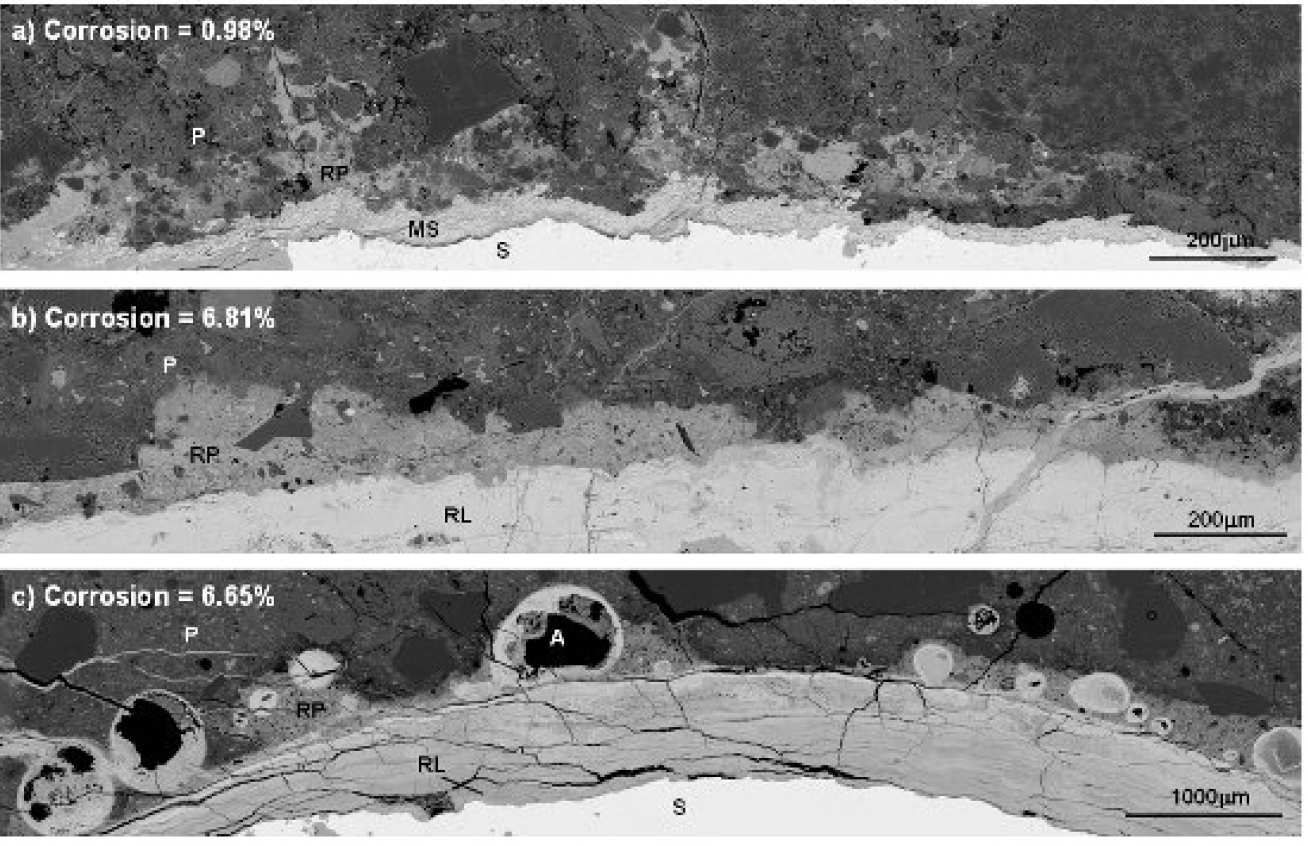
Corrosion products can migrate through the aggregate-paste interface as well as the ‘bulk’ paste. They can be deposited in cracks, air voids, inner & outer hydration products, and relicts of reacted slag. A distinct boundary between the affected and unaffected paste can be seen, indicating the extent of the rust penetration (Fig 2).
EDX microanalyses show that the affected paste has higher analysis totals, and Fe and O contents, but is depleted in Ca (Fig 3). The latter indicates dissolution of CH and/or decalcification of the C-S-H gel, which increases the local porosity and facilitates rust penetration. When pores become filled or blocked, subsequent corrosion products are forced to accumulate at the steel-concrete interface, inducing expansive pressure that leads to bond failure and cracking.
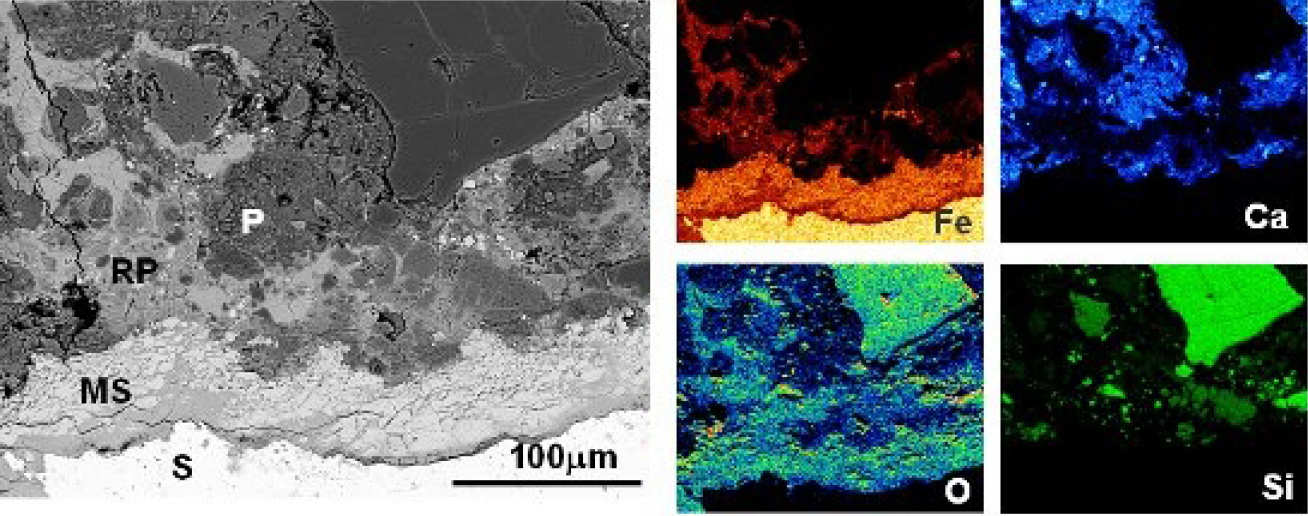
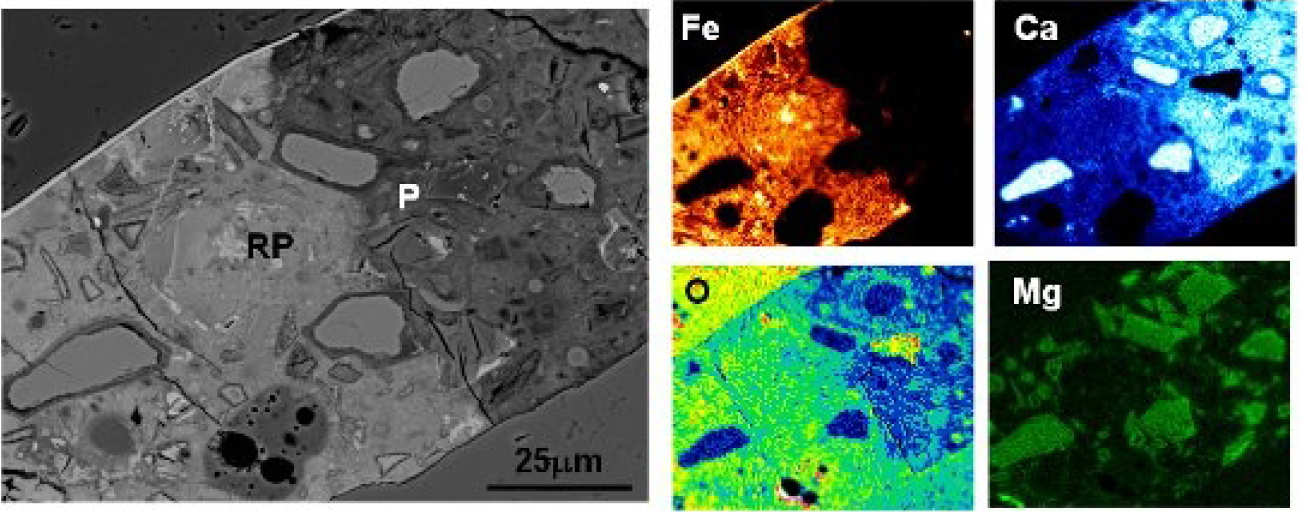
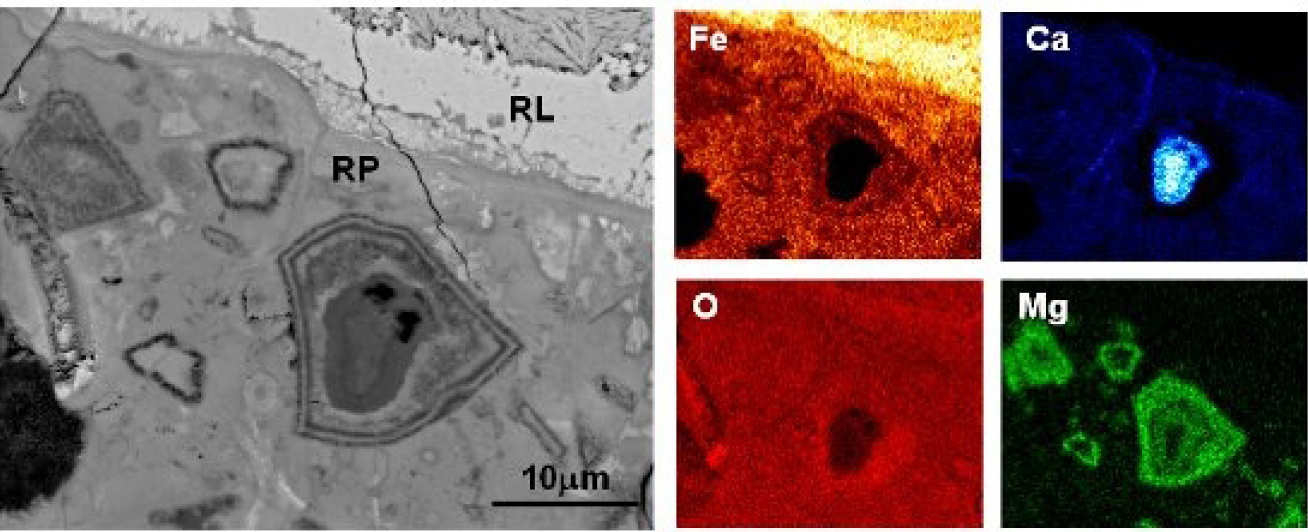
Image analysis found that only a small amount of corrosion product, approximately 100μm thick covering about 20% of the rebar perimeter, is needed to generate the first visible cover crack (~0.05mm width). Once cracking has initiated, the rust preferentially deposits in large cracks rather than pore spaces in the cement paste. Hence, the extent of rust penetration into the cement paste does not increase much with corrosion (Fig 4b).
Reference:
- H.S. Wong, Y.X. Zhao, A.R. Karimi, N.R. Buenfeld, W.L. Jin (2010), On the penetration of corrosion products from reinforcing steel into concrete due to chloride-induced corrosion, Corr. Sci., 52, 2469-2480.
- Y.X. Zhao, A.R. Karimi, H.S. Wong, B.Y. Hu, N.R. Buenfeld, W.L. Jin (2011), Comparison of uniform and non-uniform corrosion induced damage in reinforced concrete based on image analysis, Corrosion Science, 53 (9) 2803-2814
Contact us
Prof Nick Buenfeld
Tel: +44 (0)20 7594 5955
E-Mail: n.buenfeld@imperial.ac.uk