Chemical looping combustion (CLC) dates from the early 1980’s when it was proposed as a system for augmenting power station efficiency. CLC works in a simple manner: instead of performing hydrocarbon combustion in a single reaction stage, two (or conceivably more) reactions are used. An additional species is required, which re-circulates between the two reactions carrying oxygen atoms. This additional species is called the oxygen carrier and is typically a metal. As an example, consider the following two reactions using a nickel based reaction scheme
If reactions (1) and (2) are added together the nickel simply re-circulates between the two reactions, hence, from the perspective of an overall mass and energy balance, the two reactions simplify to the basic carbon oxidation reaction, thus:
Both reactions (1) and (2) are arranged to take place in separate vessels called, respectively: the oxidiser and reducer (or air reactor and fuel reactor). This leads to a key advantage of CLC: In conventional power stations, since the fuel is burned in the presence of air, the CO2 inevitably becomes diluted with nitrogen necessitating an expensive post-combustion scrubbing system. As a result, carbon capture is often seen as both energetically and economically unattractive. However, this problem is circumvented in CLC since the fuel enters the reducer rather than the oxidiser. As a result, the fuel never comes into contact with air, and therefore the CO2 produced in the system remains undiluted with nitrogen.
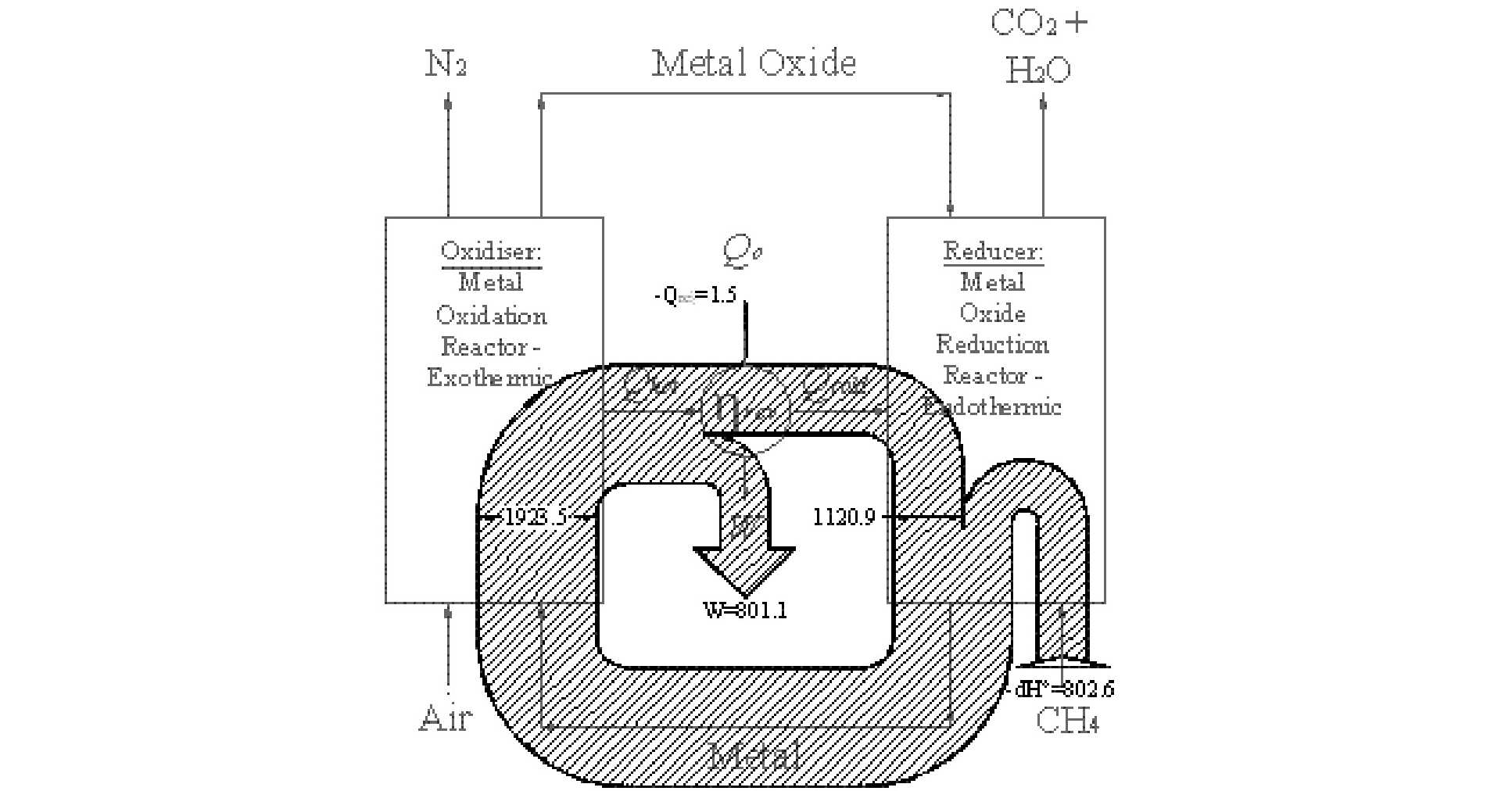
However, avoiding a post combustion scrubbing plant is not the only advantage of CLC; it can improve power station efficiency too. To illustrate this, the figure below shows a schematic diagram of a CLC plant and includes a Sankey diagram of the flow of energy. Studying the diagram below, a heat engine straddles the two reaction vessels. The oxidation reaction is arranged to be highly exothermic producing a heat flux at a high temperature – this flux is used to drive a heat engine. The heat engine, in turn, must reject heat, which it does at medium temperature into the reducer. The reduction reaction is arranged to be endothermic and it is the heat rejected by the heat engine that drives this reaction.
Why does this process enable the thermal efficiency to be increased? Careful selection of the oxygen carrying species enables the equilibrium temperature of the two redox reactions to be reduced below current metallurgical limits. Consequently, using CLC it is theoretically possible to approach a reversible IC engine without resorting to impractical temperatures. Critically, CLC achieves this increase in efficiency using the same ‘chemical plant’ that is used to capture CO2; the capital equipment used to capture CO2 is also used to increase power station performance.
Sankey diagram of energy fluxes in a reversible CLC system.
For people working in this area, please click here.