Of different fuel cell types, PEM fuel cells show the greatest promise in automotive applications due to their fast start-up and low temperature operation. PEM fuel cells convert hydrogen into electricity with the only by-product being water at an efficiency greater than an internal combustion engine. The widespread commercialisation of PEM fuel cells is currently limited by the two main barriers of cost and durability. The ESE group has a wide spectrum of expertise covering all key aspects of PEM fuel cells. The group has been working on solutions for the fuel cell challenges by innovating in electrocatalysts, fuel cell design and manufacturing, diagnostics and system integration and control, which are underpinned by multiscale modelling.
As part of the ESE group, the Titirici group at the Department of Chemical Engineering particularly focuses on the synthesis of precious-metal-free electrocatalysts and sustainable catalyst supports using various techniques with aims that include improving stability and maintaining high activity for electrochemical reactions in the PEM fuel cell. This group also works on related engineering problems, such as improving the electrochemical interface and testing setups.
Current projects
Lead researcher: Mr Yuwei Pan
PI’s: Prof. Nigel Brandon and Dr Huizhi Wang
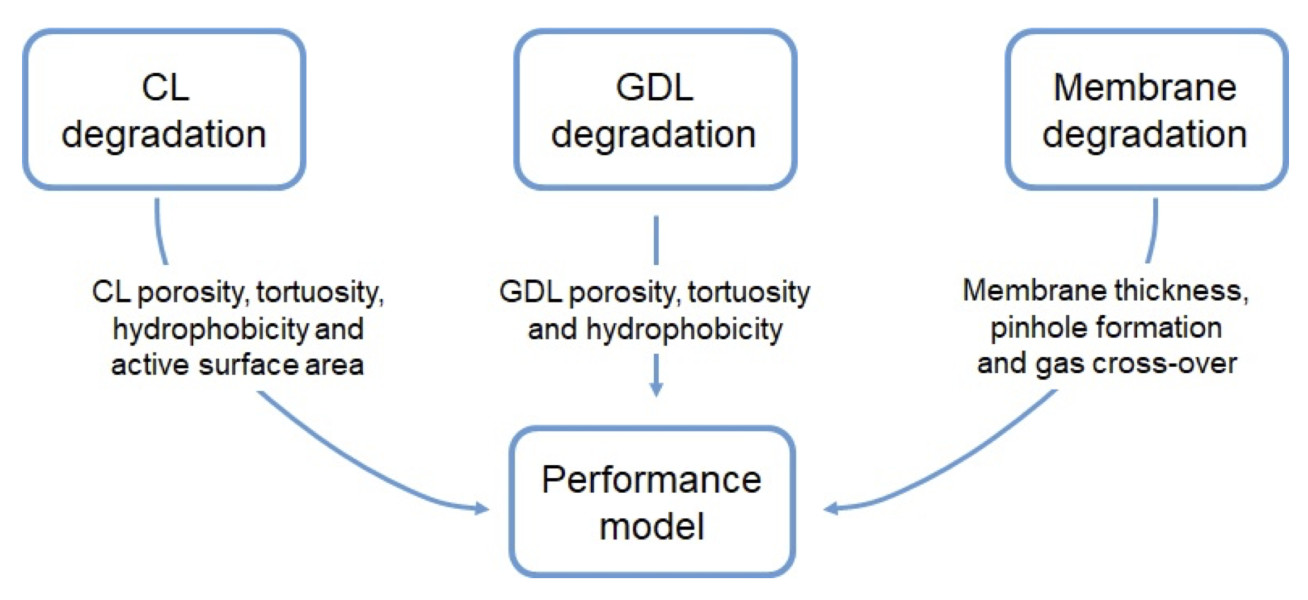
Durability is one of the major concerns of proton exchange membrane fuel cells (PEMFCs). While numerous studies have been conducted to model the ageing phenomena at different time and length scale, a comprehensive multi-scale model with multiple mechanisms is still missing. The objective of this research is to build a cell to stack level degradation model with pore-scale ageing phenomena of all components. To achieve this goal, this study will contain four stages: continuum cell-level model, pore-scale ageing model, multi-scale coupling strategies and stack performance decay model. At the end of this work, it is expected to form a multi-scale simulating platform for PEM fuel cell degradation, which could potentially aid the investigation of ageing mechanisms, enhancement the system design and optimization of control strategies.
Publications:
-
Pan, Y, Wang, H, Brandon, NP, 2021, Gas diffusion layer degradation in proton exchange membrane fuel cells: Mechanisms, characterization techniques and modelling approaches, Journal of Power Sources, Vol: 513, Pages: 230560.
- Pan, Y., Wang, H. and Brandon, N.P., 2022, A fast two-phase non-isothermal reduced-order model for accelerating PEM fuel cell design development, International Journal of Hydrogen Energy, Vol: 47(91), Pages: 38774-38792.
Recent publications 2020 - to date
Pan, Y., Wang, H. and Brandon, N.P., 2022, A fast two-phase non-isothermal reduced-order model for accelerating PEM fuel cell design development, International Journal of Hydrogen Energy, Vol: 47(91), Pages: 38774-38792.
Pan, Y, Wang, H, Brandon, NP, 2021, Gas diffusion layer degradation in proton exchange membrane fuel cells: Mechanisms, characterization techniques and modelling approaches, Journal of Power Sources, Vol: 513, Pages: 230560.
Jiao K, Xuan J, Du Q, Bao Z, Xie B, Wang B, Zhao Y, Fan L, Wang H, Hou Z, Huo S, Brandon NP, Yin Y, Guiver MD, 2021, Designing the next generation of proton-exchange membrane fuel cells, Nature, Vol: 595, Pages: 361–369.
Boldrin P, Malko D, Mehmood A, Kramm UI, Paul S, Weidler N, Kucernak A, 2021, Deactivation, reactivation and super-activation of Fe-N/C oxygen reduction electrocatalysts: gas sorption, physical and electrochemical investigation using NO and O2, Applied Catalysis B: Environmental, In press
Favero S, Stephens IEL, Titirici MM, 2021, Engineering the Electrochemical Interface of Oxygen Reduction Electrocatalysts with Ionic Liquids: A Review. Advanced Energy and Sustainability Research, Vol: 2(1), Page: 2000062.
Xie B, Zhang G, Jiang Y, Wang R, Sheng X, Xi Fu, Zhao Z, Chen W, Zhu Y, Wang Y, Wang H, Jiao K, 2020, “3D+1D” modeling approach toward large-scale PEM fuel cell simulation and partitioned optimization study on flow field, eTransportation, Vol: 6.
Jorge AB, Jervis R, Periasamy AP, Qiao M, Feng J, Tran LN, Titirici MM, 2020, 3D Carbon Materials for Efficient Oxygen and Hydrogen Electrocatalysis, Advanced Energy Materials, Vol: 10, Page: 1902494.
Wang B, Zhang G, Wang H, Xuan J, Jiao K, 2020, Multi-physics-resolved digital twining of proton exchange membrane fuel cells with a data-driven surrogate model, Energy and AI, Vol: 1, Page: 100004