Key information
We are looking for participants with Alzheimer's disease to take part in a non invasive brain stimulation study. The study involves 16 visits to the Imperial College London White City campus, during which you will be asked to complete a series of cognitive tasks while we stimulate the hippocampus and record brain activity.
For further information and to get involved, please contact Research Assistant Kaarin Sabad (k.sabad@imperial.ac.uk).
Alzheimer's disease study
Summary of the study
We are looking for participants with Alzheimer's disease or Mild Cognitive Impairment (65+) to take part in a study investigating the use of non-invasive deep brain stimulation and its effects on the hippocampus, a structure deep within the brain that is responsible for memory function and often the first area where the disruptions of normal brain activity occur in Alzheimer's disease.
The purpose of this study is to assess the short-term effects of temporal interference (TI) stimulation of the hippocampus on the brain activity and cognitive performance of people with early stage Alzheimer's disease.
What does the study involve?
After expressing interest, you will be invited for a telephone pre-screening call to determine your initial suitability for the study. If successful, you will be enrolled and invited to attend a screening visit and 16 study visits spanning 3 months at Imperial College London's Clinical Research Facility (ICRF) at Hammersmith Hospital.
Screening visit
Before commencing on the study, you will be invited for a screening visit at the ICRF, with the aim to determine your eligibility for the study. At the screening visit, a detailed description of the study will be provided and any questions you may have will be answered. You will then be familiarised with the technology we use to measure your brain activity and stimulate the brain. This will be followed by an interview with the lead clinician on our study.
Study visits
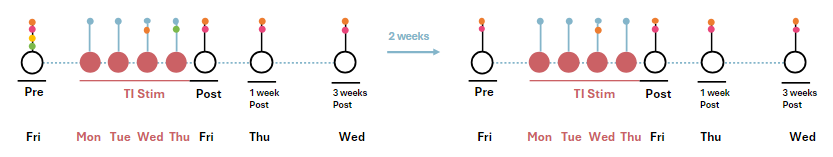
The study will consist of 16 visits spanning 3 months. This includes 2 weeks of TI stimulation separated by a 6-week gap including a 1-week and 3-week follow-up assessment. The two stimulation weeks are also preceded by a baseline assessment. See image below for a visual representation of the study schedule.
Baseline assessment visits
The baseline assessment visits take place on the Fridays preceding the two stimulation weeks. These visits will consist of cognitive tasks, during which the activity of the brain will be recorded with EEG (electroencephalography) and a clinical assessment. On the first baseline visit, a blood sample and an MRI recording will additionally be obtained. The first baseline visit will take 5.5 hours and the second 4 hours in total.
Stimulation visits
The TI stimulation visits will take place on Monday-Thursday 6 weeks apart. During the visits, you will be stimulated with TI and you brain activity will be recorded with EEG while you perform cognitive tasks. The stimulation visit consists of 2 separate 45-minute stimulation sessions separated by a lunch break. On the last day (Thursday), a blood sample will be obtained. Each visit will take approximately 4 hours to complete.
Post assessment visits
On each Friday at the end of the stimulation week, there will be a post assessment visit. As with the baseline visit, you will be asked to perform some cognitive tasks while your brain activity is recorded with EEG after which a clinical assessment will be conducted. These visits will last approximately 3.5 hours.
Follow-up visits
Each stimulation will be followed by a 1-week and 3-week follow-up visit. In these follow-up visits, you will again be asked to perform cognitive tasks while an EEG recording of your brain is obtained and we will perform some clinical assessments. These visits will last between 2 to 3 hours.
Am I eligible to take part?
You will be able to take part in the study if:
- You are aged 65-100 years
- You have a diagnosis of Alzheimer's disease or Mild Cognitive Impairment and have been experiencing difficulties with your memory
- You have not suffered from traumatic brain injury, epilepsy, strokes or frequent migraines
- You have no metal or electronic objects in your body, such as a pacemaker
- You do not have any severe skin lesions on our scalp
- You do not suffer from anxiety in confined spaces (claustrophobia) such that you cannot tolerate an MRI scan
Will I be reimbursed for taking part?
Travel to and from the study site will be arranged by the study team and you will receive a £10/hour for each study visit. Lunch will be provided by the study team on the days that it is required.
How can I get involved?
Please email Research Assistant Kaarin Sabad (k.sabad@imperial.ac.uk) to express your interest.
General enquiries
Dr Nir Grossman
Senior Lecturer in Dementia Research and Group Leader at the UK DRI
nirg@imperial.ac.uk
+44 (0)20 7594 6805
