Biomedical Imaging Agents and Technologies
This works involves rational probe design, via small molecules or functionalised nanoparticles, to effect new and improved biomedical (PET, MRI, optical, ultrasound) imaging agents that has significant impact on the early detection of disease and the evaluation of medical treatment.
Biomedical Imaging Agents and Technologies
- Functionalised Iron Oxide Nanoparticles and Functionalised Microbubbles for Cancer Imaging
- Dual-Modal MRI/Optical Imaging Probes
- Development of Chemical Methodologies for 11CO PET Radiolabelling
- Dual-Modal and -Functional Metal Chelates for Biomedical Imaging: Zinc-Sensing for Diabetes Diagnosis
- Imaging Cell Death via 18F and 68Ga PET Radiolabelling
In collaboration with Prof. Eric Aboagye (Surgery and Cancer), we are developing novel MRI contrast agents for cancer imaging, comprising superparamagnetic iron oxide nanoparticles (SPIOs) conjugated to peptide ligands of CXCR4, which is overexpressed in tumours. We have devised a novel and patented process whereby the nanoparticles ‘self-assemble’ in the body to increase the normally poor sensitivity, as well as being targeted to the required areas i.e. tumours. This work demonstrates the potential of CXCR4 targeting together with MMP trigger and copper-free click chemistry for efficient and more sensitive cancer MRI in vivo. This work will help to pave the way towards the development of further ‘smart’ targeted nanoparticles and nanomedicines for a variety of diseases. We have recently developed 68Ga-labelled microbubbles for dual-modal PET/US imaging.
Key references: Chem. Sci., 2019, 10, 5603; Angew. Chem. Int. Ed., 2018, 57, 5808; Nanoscale, 2017, 9, 11318; Angew. Chem. Int. Ed., 2014, 53, 9550; J. Mater. Chem., B, 2014, 2, 868; Dalton Trans., 2014, 43, 5535; Chem. Soc. Rev., 2013, 42, 7816; Am. J. Nucl. Med. Mol. Imagaing, 2013, 3, 372

In recent years there have been increasing reports of multimodal imaging probes whereby two or more imaging modalities are combined. The combination of modalities leads to probes which give enhanced visualization and improved reliability of data by synergistically combining imaging techniques to overcome their inherent disadvantages. MRI gives high anatomical resolution and deep tissue penetration, but lacks sensitivity. Optical imaging however, boasts high sensitivity but has limited tissue penetration. The combination of the two techniques yields a probe which is able to provide a more complete picture of the biological area of interest. There are few reports of dual-modal MRI/optical imaging agents in the literature that are not of nanoparticle nature, and we have recently reported two novel methods for conjugating fluorescent rhodamine derivatives to DO3A, a small aza-macrocycle. We evaluate the two rhodamine-DO3A Gd(III) derivatives as multi-modal MRI/optical imaging agents by measuring their relaxivity and fluorescence properties, as well as their in vitro tumour cellular localization. The Tb(III) analogues were synthesized to assess their dual-luminescence properties, showing that the organic and the metal-based luminescence can be separated on different timescales.
Key references: Nat. Commun., 2019, 10, 1420; Nanotheraostics, 2017, 1, 186; Inorg. Chem., 2013, 52, 14284
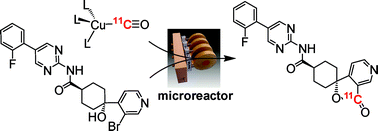
Processing and reacting short-lived radioactive gases is an extremely challenging area of synthetic chemistry. This problem is further exacerbated when those gases are poorly reactive. Our approach to this 11CO reactivity problem was to devise a chemical trapping method to enhance 11CO reactions and avoid the need for complex and specialised equipment. Our trapping and reaction method is based on using inorganic complexes that contain transition metals that are uniquely capable of sequentially trapping, concentrating and then releasing 11CO from a gas stream. It is anticipated that the technical simplicity of this method coupled with its efficiency will make 11CO chemistry more readily accessible to non-specialists. In collaboration with Prof. Tony Gee (King’s College, London), we are now using the technique to form a range of 11C labelled biomolecules for PET imaging ready for the clinic.
Key references: Chem. Commun., 2017, 53, 2133; Eur. J. Inorg. Chem., 2014, 1896; Dalton Trans., 2012, 41, 83; Chem. Eur. J., 2011, 17, 460; Org. Biomol. Chem., 2011, 9, 3313; J. Label. Radiopharm., 2011, 54, 135; Adv. Synth. Catal., 2009, 351, 3260; Chem. Commun., 2009, 3696
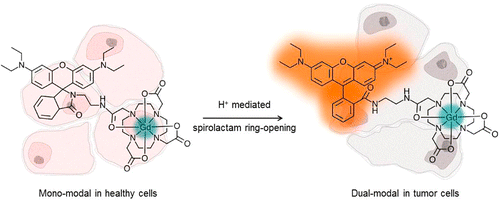
In this work, via collaboration with Prof. Guy Rutter (Medicine), we have formed a series of dual-modal MR/NIR fluorescent zinc sensing probes for diabetes imaging, in order to advance the area of molecular imaging. Despite the contribution of changes in pancreatic beta cell mass to the development of all forms of diabetes mellitus, few robust approaches currently exist to monitor these changes prospectively in vivo, restricting our understanding of disease aetiology and opportunities for personalized treatment. Whilst magnetic resonance imaging (MRI) provides a potentially useful technique, targeting MR-active probes to the beta-cell has proved challenging. Zinc ions are highly concentrated in the secretory granule, but relatively less abundant in the exocrine pancreas and in other tissues. We are therefore developing functional dual-modal probes based on transition metal chelates capable of binding zinc.
Key references: Chem. Commun., 2018, 54, 3227; Dalton Trans., 2015, 44, 4476; Chem. Eur. J., 2015, 21, 5023

Mitochondrial dysfunction is firmly linked to a number of disease states including those of cardiometabolic, oncologic and neurodegenerative origin such as cardiovascular disease, cancer, Parkinson’s disease and dementia. Although seemingly unrelated, these conditions share common factors, such as the production of reactive oxygen species, genetic variants, aging and a reduction in mitochondrial biogenesis, which all contribute to mitochondrial dysfunction and the development of disease pathology. Positron Emission Tomography (PET) is a non-invasive imaging approach that allows the visualisation and quantification of biological processes at a molecular level. Lipophilic cations, which accumulate in mitochondria due to the electrochemical membrane potential, provide a potential target for novel PET probe development. Indeed, recent developments support the application of labelled lipophilic cations as imaging probes of apoptosis in oncology. In collaboration with Imanova Ltd., we have developed novel fluorine-18 labelled lipophilic cations, that have demonstrated encouraging results in vitro as imaging agents that sense changes in mitochondrial membrane potential in various bodily organs.
Key references: J. Nucl. Med., 2019; Dalton Trans., 2018, 47, 15448 ; Mol. Pharm., 2014, 11, 3818 ; J. Label. Radiopharm., 2013, 56, 313
Contact
Professor Nick Long
Email: n.long@imperial.ac.uk
Telephone: +44 (0)20 7594 5781
Location
501J
Molecular Sciences Research Hub
White City Campus


