Funded by the Wellcome Trust, The MedTech Accelerator helps de-risk Imperial College MedTech projects which have a high translational potential.
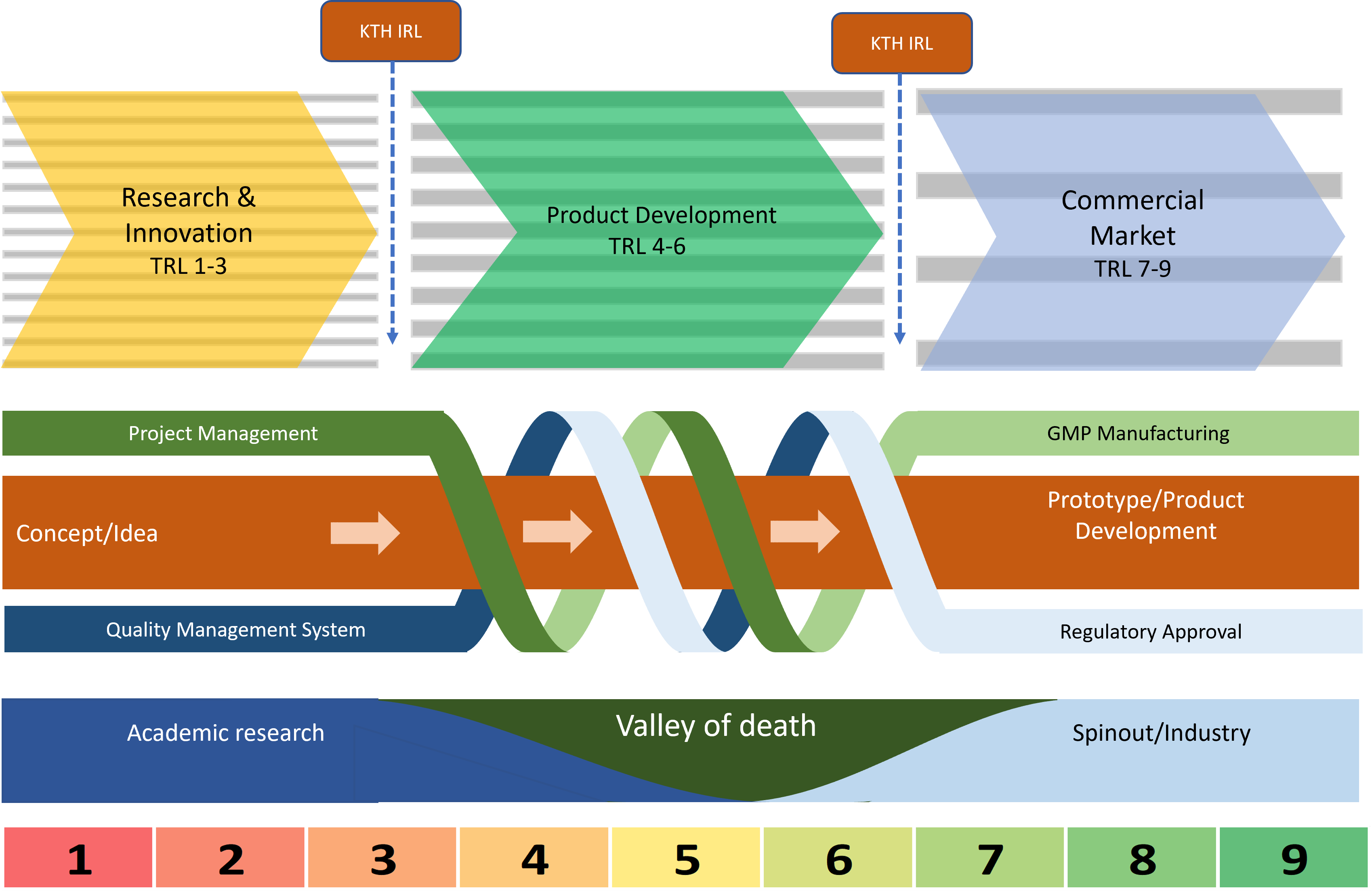
We run competitive calls for managed funding (see below), for MedTech projects with high translation potential. The Accelerator focuses on increasing the technology readiness levels of pre-clinical selected research & development projects developing medical technology innovations at Imperial College. But this can not be pursued in isolation of customer and market research - the technology must be attractive to a customer and answer an unmet clinical need. Plus, securing further funding is key to supporting the lengthy medtech development journey, and in MedTech, we also have to consider the Regulatory and reimbursement barriers, the development team needs to understand how this may affect technical decisions. Finally, development of entrepreneurship skills is key to success.
Aligning all of those factors is quite complex, especially for advanced technologies arising for years of research, and also because these factors are dependent of each other’s. The good news is that there is a lot of support for MedTech Entrepreneurs at Imperial College, including the MDDE MRes, BSC in Biomedical Technology Ventures, MedTech SuperConnector, NIHR BRC, NIHR London IVD Cooperative, and of course the other Wellcome-funded streams of support at MedTechOne.
During the Accelerator programme, considering those factors, we help selected MedTech project teams establish their development action plan, including TRL based milestones, and in close discussion with the technology transfer office (the Industry Partnership and Commercialisation team), with the ultimate goal of increasing the attractiveness of the proposition and ability for the team to engage with potential customers, and funding application goals.
We do this through our project management function: we navigate the projects we support through the medtech translation journey, signposting to internal and external programmes at the appropriate time We also have quality/regulatory expertise and a Quality Management System (QMS) (shown in Figure 2).