Modelling structure-activity relationships in polymer photocatalysts
The work has provided computationally efficient tools to model the structure and electronic properties of molecular electronic materials, to design new materials and to understand the behaviour of such materials when applied to solar energy conversion and charge storage. In the case of polymer photocatalysts, we combined different modelling tools to show that the interactions between the polymer and its liquid environment control the photocatalytic activity, by controlling the likelihood of the first step in the photocatalytic process where a polymer that has been excited by absorbing light transfers a charge to a molecule in the surrounding solvent (Figure 1). The work could explain why polymers that contain polar components outperform those that do not. We used this understanding to design better performing materials. Previous approaches had not considered the polymer-solvent interactions.
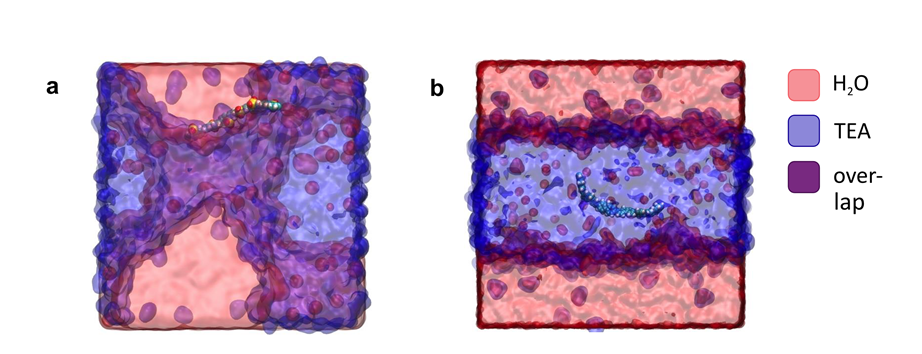
Molecular dynamics simulations and energy level calculations in water and mixed water/TEA environments. Snapshots of atomistic molecular dynamics simulations of oligomers of (a) a polar polymer P10 and (b) a non-polar fluorene polymer both in a mixture of TEA (blue) and water (red).
Non-radiative energy losses at interfaces
In a separate study we developed a new model of charge recombination at a molecular interface, something which is relevant to both photovoltaic and photochemical solar energy conversion. We discovered that the non-radiative recombination, the main loss pathway in molecular materials, is controlled partly by the brightness of the excited states at the interface, and that this can in turn be controlled by choosing the energy levels of the materials. This represents a design rule to bring molecular solar energy conversion closer to the ideal limit. By adjusting the energies of the states involved in recombination, their brightness, and the strength of coupling to vibrational modes, the rate of energy loss through non-radiative recombination could be suppressed, improving the efficiency of molecular solar cells (Fig. 2).
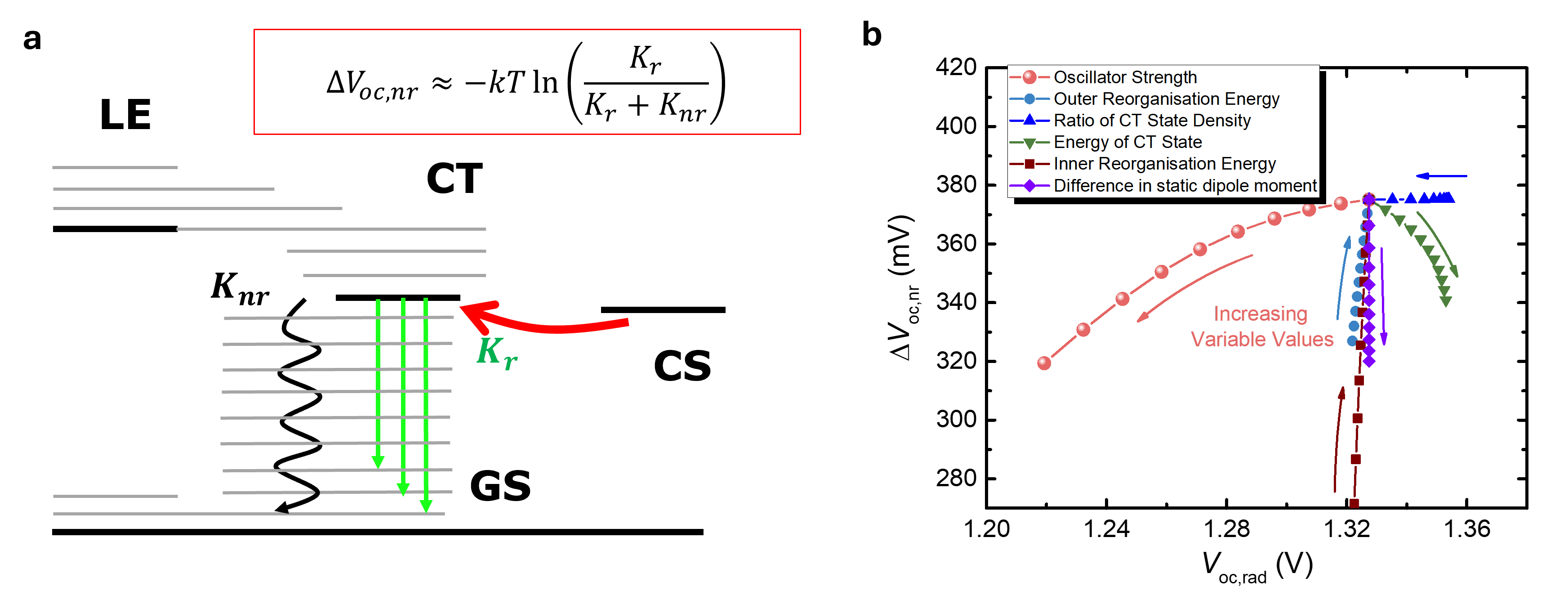
Non-radiative energy losses at organic heterojunctions. (a) State diagram showing radiative and non--radiative decay pathways via an interfacial charge transfer (CT) state. The non-radiative loss can be expressed as a voltage loss. (b) Calculation of the effect of varying molecular parameters on the non-radiative voltage loss.
Polymer battery device
Regarding electrochemical energy storage, we demonstrated a novel battery device using conjugated polymers with polar side chain that can transport and store ions (Figure 3). The device could charge and discharge very rapidly compared to state-of-the art lithium ion devices and operates in a safe, salt-water electrolyte. Our research addressed the mechanical stability of the electrode materials and using multiscale modelling and experiments we showed that whereas polar groups are needed for the electrodes to charge in aqueous electrolytes, the mechanical stability is greatly helped by introducing a small fraction of non-polar side groups. An all-polymer version of the device was then developed using a bio-derived polymer for the electrolyte which shows fairly robust performance.

Polymer battery device (a) schematic of the battery device based on conjugated polymer electrodes with a salt-water electrolyte. (b) reactions that occur at the anode and cathode under changing and discharging.
Modelling structure-property relationships in conjugated polymers
All these applications were underpinned by basic research into the relationship between polymer structure and the material’s electronic properties. In one important development we showed that ordered packing of polymer chains is not necessary for the material to show good electronic transport properties. In fact, for stiff and linear conjugated polymer chains, the highest electronic mobility is achieved in structures where chains cross at right angles, and this can be designed in via the polymer’s chemical structure. This introduced a new design approach for high performance conjugated polymers (Fig. 4).
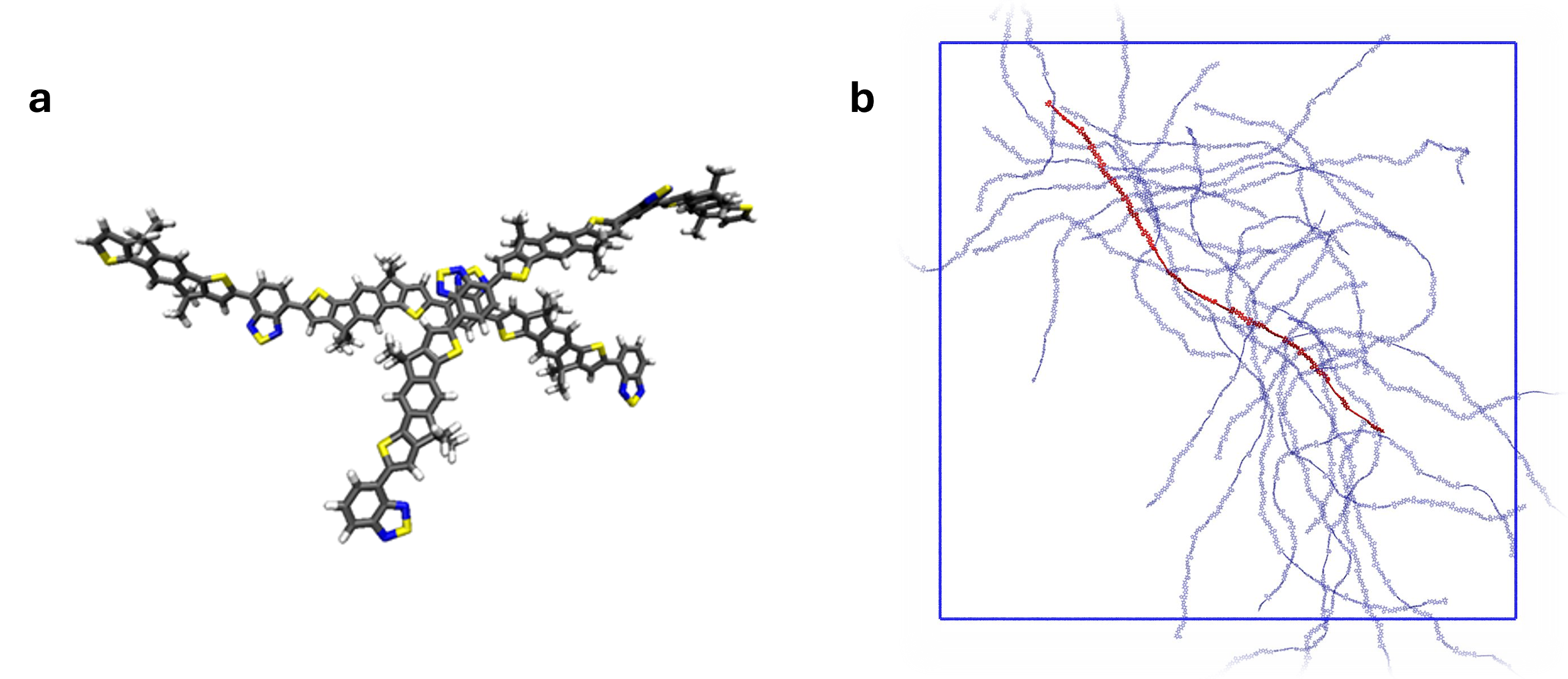
Connectivity in C16IDTBT (a) Detail showing perpendicular crossing motif of two IDTBT polymer chains (b) Snapshot of MD simulation showing connectivity between 24-mers of C16-IDTBT in the solid state.