
GRACE Trial Co-ordinating Centre
Section of Vascular Surgery
Room 4E 16, 4th Floor East Wing
Charing Cross Hospital
Fulham Palace Road
London W6 8RF
Email: gracetrial@imperial.ac.uk
Funder
National Institute for Health Research (NIHR) – Health Technology Assessment (HTA).
Research aim
To evaluate the potential benefit of Graduated Compression Stockings (GCS) in addition to extended duration pharmacological thromboprophylaxis (EDPTP) for surgical patients at highest risk of venous thromboembolism (VTE).
Background
Hospital-acquired thrombosis (HAT) is defined as any venous thromboembolism (VTE) during admission and within 90 days of hospital discharge, and is a term that encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE). It is estimated that as many as 60% of all VTE cases are hospital related. HAT represents a significant cause of preventable mortality, with as many as 60.4 deaths from VTE per 100,000 hospital admissions reported in the year 2020-2021 within the NHS – equivalent to 12,303 people dying each year from hospital-caused VTE, which is 10-fold higher than the number dying from road traffic accidents. The UK annual VTE-related mortality prior to implementation of a national programme of thromboprophylaxis, was estimated to be up to 32,000 fatalities, with associated costs as high as £640 million per annum.
VTE is a significant cause of long-term disability with subsequent societal and economic consequences. Morbidity following a DVT is substantial, with as many as 50% of patients developing post thrombotic syndrome (PTS), characterised by troubling chronic leg pain, oedema, and venous skin changes. Crucially, the severity of chronic venous disease is much worse in those with PTS in comparison to primary venous disease and the rate of venous ulceration is as high as 29%. Those with PTS have a significantly worse quality of life, which is comparable to those with cancer and congestive heart failure. Furthermore, PE is associated in some with life-long functional, physiological and psychological harm. Ultimately, PE can cause death during the index event and in an estimated 2% can be complicated by chronic thromboembolic pulmonary hypertension, resulting in chronic pulmonary hypertension and heart failure. Persistent cardiac insufficiency have been reported in as many as 13.2% of PE cases.
Surgery is a significant risk factor for VTE, with those undergoing lower extremity orthopaedic operations, major oncological surgery or pelvic surgery being at the highest risk. Previous studies report the risk of untreated high-risk surgical patients developing HAT is as high as 40-60% in elective joint replacement and 15-40% in general surgery patients. For these patients at highest risk of VTE, the prevention strategies include extended pharmacological thromboprophylaxis (EDPTP) prescribed beyond the hospital stay and provision of graduated compression stockings (GCS). National Institute for Health and Care Excellence (NICE) [NG89] guidelines recommends certain cohorts of surgical patients should receive mechanical thromboprophylaxis, most commonly in the form of GCS, in addition to the EDPTP. This cohort encompasses patients undergoing orthopaedic surgery with ongoing lower limb immobilization including elective hip replacement surgery (EDPTP for 28-38 days post-surgery) and elective knee replacement surgery (EDPTP for 14 days post-surgery) in addition to those undergoing major cancer surgery in the abdomen or pelvis (EDPTP for 28 days post-surgery).
There is currently no high-quality evidence to support the use of GCS in addition to EDPTP in patients deemed to be at the highest risk of VTE. A Cochrane Review which reported benefit for GCS included small trials (18-440 patients) only one of which was published this millennium, and many of these trials were funded by stocking manufacturers. Additionally, two large recent trials were excluded from this review in stroke and orthopaedic patients - neither study supported the use of GCS. Furthermore, the NIHR GAPS trial reported that pharmacological thromboprophylaxis alone is non-inferior to pharmacological thromboprophylaxis and GCS. This proposal includes individuals requiring EDPTP, at highest risk, who were excluded from GAPS, hence the results cannot be directly extrapolated. In preparation for this study, a systematic review and meta-analysis of VTE rates in surgical patients treated with pharmacological thromboprophylaxis in addition to GCS in comparison to pharmacological thromboprophylaxis alone was undertaken in accordance with PRISMA guidelines. Overall, the additional benefit of GCS is not clear based on existing data.
Design
Assessor-blinded randomised controlled trial with a non-inferiority comparison using an intention-to-treat analysis
Setting
This trial will take place in hospitals (up to 50 sites).
Inclusion/exclusion criteria
Inclusion criteria
- Adults (≥18 years of age)
- Participants undergoing surgery; risk assessed as requiring EDPTP*
Exclusion criteria
- Contraindications to EDPTP or GCS
- Individuals requiring therapeutic anticoagulation e.g., anticoagulation for previous DVT
- Known thrombophilia or thrombogenic disorder
Target population: N=8608 (Approx 200 participants per site over 24 months)
*Examples of procedures from which patients are at highest-risk of VTE and usually require extended duration pharmacological thromboprophylaxis (EDPTP) include, but are not limited to: orthopaedic surgery (total hip replacement, total knee replacement, colorectal surgery (colectomy, splenectomy), upper gastrointestinal surgery (oesophagectomy, gastrectomy), urological surgery (cystectomy, nephrectomy), and gynaecological oncology surgery (radical hysterectomy, radical trachelectomy).
Outcome measures
The primary Imaging-confirmed lower limb DVT with or without symptoms, or PE with symptoms, occurring up to 90 days post-surgery.
Secondary outcomes
- Mortality
- Adverse events with GCS
- Adherence with GCS
- Adherence with EDPTP
- Safety outcome measures
Follow up
Participants will also undergo a full lower limb deep venous thrombosis scan at 21- 35 days at the recruiting site.
Participants will also be followed-up centrally by the coordinating centre Imperial College London (by telephone, online or SMS) at 7-days, between 21 and 35 days and 90-days.
Timelines
The study will commence on 1st January 2024. The recruitment period is 2 years.
Patient video
GRACE - Staff Training
GRACE Staff Training and forms.
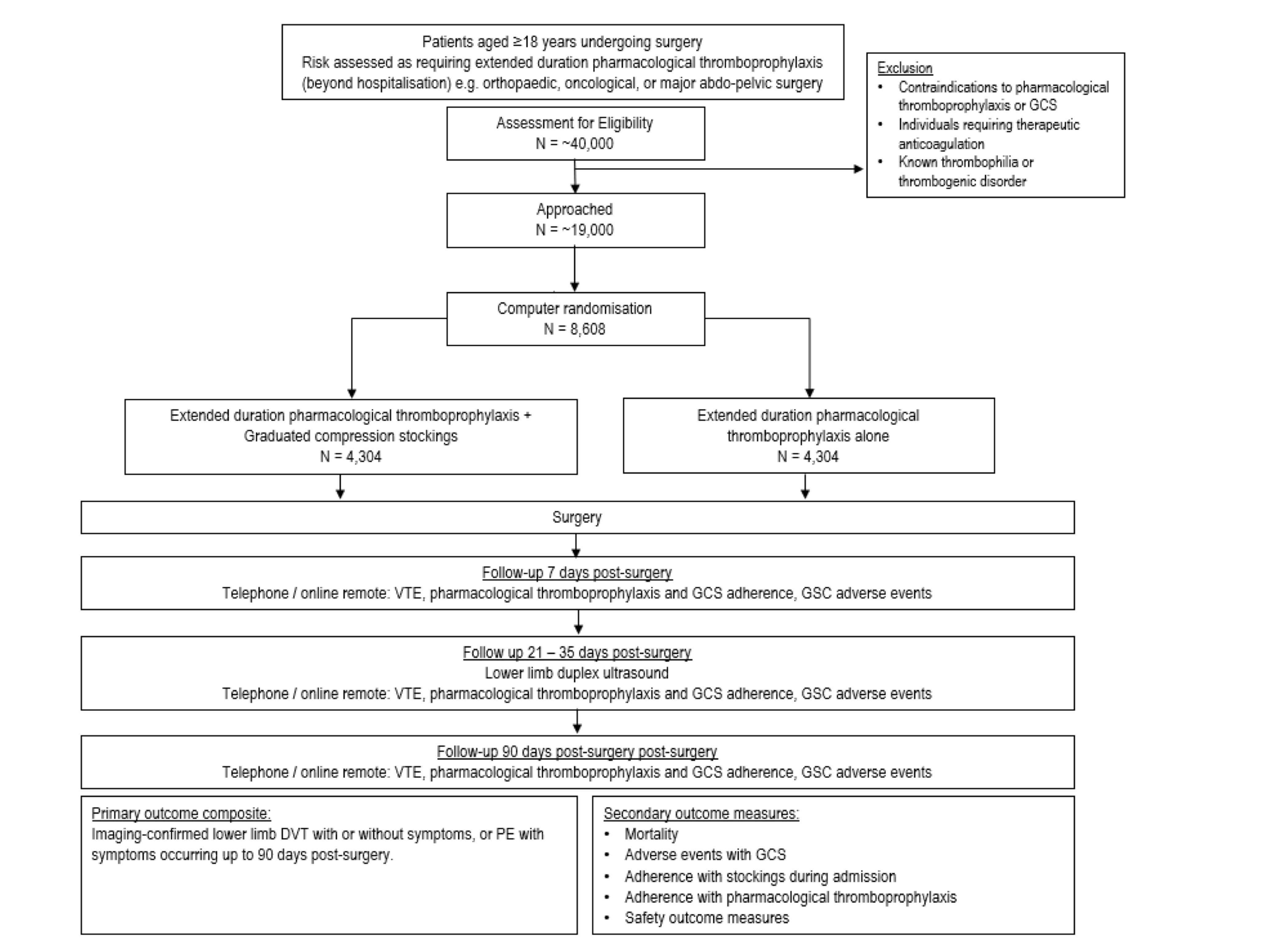
Figure 1: GRACE Flow-chart
NIHR Associate PI Scheme
The Associate Principal Investigator Scheme aims to develop doctors, nurses and other health professionals to become the Principal Investigators (PIs) of the future. For more information on how the scheme works and to register to become an API, follow this link.
Trial co-ordinating centre staff
Professor Alun Davies
/prod01/channel_3/media/images/people-list/AHD2.jpg)
Professor Alun Davies
Chief Investigator
Dr Francine Heatley
/prod01/channel_3/media/images/people-list/Francine-Heatley-copy.png)
Dr Francine Heatley
Clinical Trials Manager
Rebecca Lawton
/prod01/channel_3/media/images/people-list/Rebecca-Lawton.jpeg)
Rebecca Lawton
Clinical Trials Manager

This study is funded by the National Institute for Health Research (NIHR) – Health Technology Assessment (HTA). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
