Contact
Commonwealth Building, 10th Floor
Hammersmith Campus,
Du Cane Road,
London, W12 0NN
What we do
Our group leverages state-of-the-art induced pluripotent stem cell (iPSC) technology to improve the understanding of liver physiology and disease. We generate iPSCs from patients and combine this with bioengineering and gene editing to generate cell, organoid and animal models. We also apply this approach toward the creation of novel cell therapies. Finally, to better understand which patients are most likely to benefit from such therapies, we leverage disease registries and electronic health records to precisely characterise the natural history of disease.
Why it is important
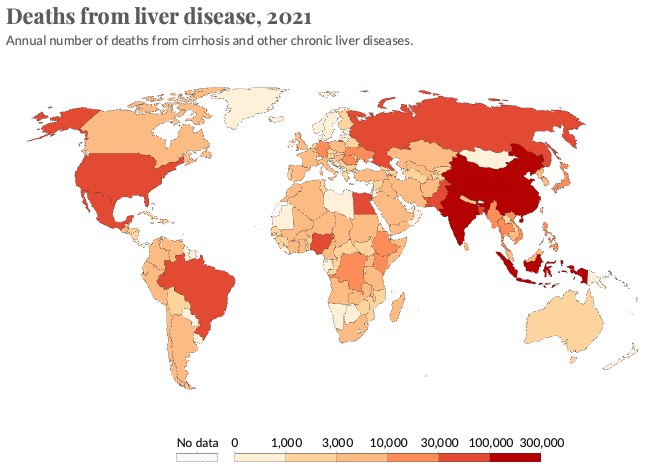
The liver is a large and complex organ. Its functions are essential to life. Some of these functions include carbohydrate, fat and protein metabolism, drug detoxification and bile excretion. Because of its vital regulatory function, disruption to the liver can lead to liver failure and death. Liver disease accounts for approximately 2 million deaths per year worldwide and since 1970s the deaths due to liver disease have increased by 400%. Currently, the only curative treatment for patients with liver failure is orthotopic liver transplantation, so alternative treatment options are urgently required. However, research into liver diseases, including studies of the molecular pathological mechanisms and research into novel and more effective therapeutics, is hindered by a lack of hepatic models that can faithfully recapitulate complex disease phenotypes. Therefore, there is a strong need to generate better models, which is what our lab does using iPSCs and human tissue. In addition, the ability to generate patient-specific iPSCs, and their unlimited self-renewal ability, makes iPSC-derived hepatocytes a potentially good source for future cell therapy.
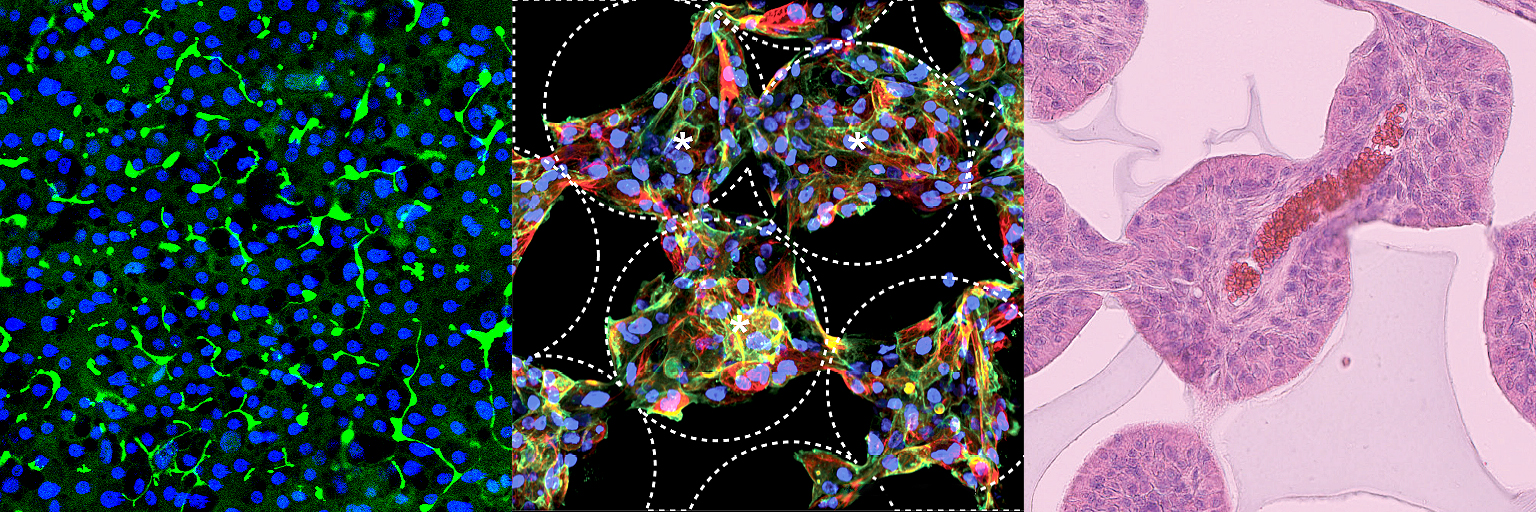
How it can benefit patients
Our iPSC-hepatocyte models allow us to study the molecular mechanisms underpinning liver disease. We use this understanding to discover more effective therapeutics for patients with these diseases.
Modelling human disease in the lab
A lack of easy-to-use, scalable, cheap models that accurately pheno-copy human disease is a major challenge holding back the development of new therapies and one that has not been addressed to date by more conventional model systems such as the mouse. As a result, the field has turned its attention to (i) patient-derived induced pluripotent stem cells (ii) organoids (ii) humanized mice and (iii) ex vivo whole organ/tissue slice perfusion systems as alternatives. These technologies require access to appropriately consented patient material and can be highly resource intensive (person-power + consumables). Despite these challenges, our lab is fully equipped with the necessary expertise and facilities to utilise this extensive range of modelling techniques, offering a comprehensive platform for disease modelling and therapeutic development.
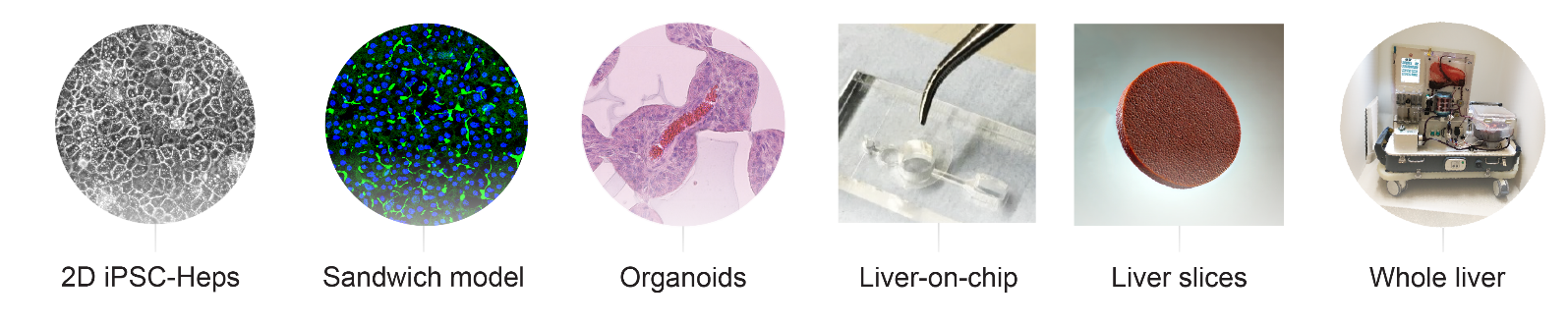
Integrating In Vivo and In Silico Approaches
To enhance the fidelity and applicability of our disease models, our laboratory also integrates in vivo and in silico methodologies. This approach facilitates the validation of experimental models against real-world clinical data, while enabling their refinement through computational simulations. Our in silico analyses are bolstered by rich datasets, including comprehensive Electronic Health Records (EHR) and detailed sequencing data (single-cell RNA seq). This integration allows for a deeper understanding of genotype-phenotype correlations and elucidates the mechanisms of action of potential therapeutics.
Therapeutic Potential of iPSC-Hepatocytes
iPSC-hepatocytes not only serve as valuable models for disease study but also hold promise as direct therapeutic agents. These highly active cells can potentially be used to treat a variety of liver diseases by replacing damaged tissue and restoring liver function. This therapeutic potential leverages the ability of iPSC-hepatocytes to function like native liver cells, offering a regenerative medicine approach that could transform the treatment landscape for a wide range of liver conditions.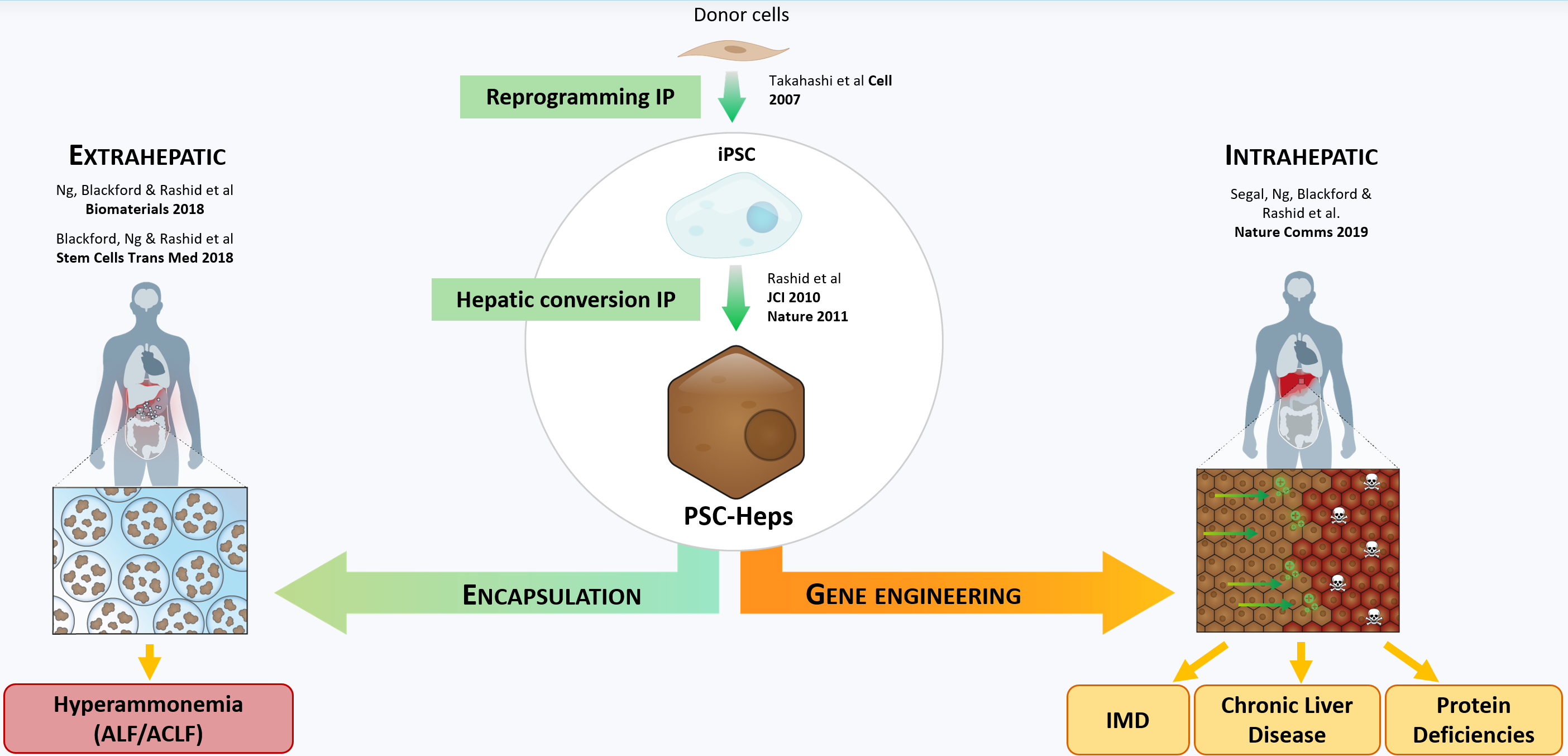
Training in the Lab
The Rashid Lab offers comprehensive training programs for a diverse range of scholars including postdoctoral fellows, graduate students, clinical fellows, medical students, and undergraduate students. Trainees benefit from personalised one-on-one mentoring sessions tailored to their individual research goals. Additionally, our team convenes weekly to review progress and discuss ongoing projects, fostering a collaborative and supportive environment.
Faculty, fellows, students, and staff also participate in weekly seminars hosted by the Department of Metabolism, Digestion, and Reproduction. These sessions are integral for interdisciplinary learning and networking with other research groups.
More info
PhD studentships
Research Fellowships
- Imperial College Research Fellowships
- UKRI FLF
- MRC Daphne Jackson fellowship
- Wellcome Early-Career Awards
Clinical Fellowships
- Imperial Clinical Academic Training Office
- MRC Predoctoral clinical research training fellowship
- MRC Clinician scientist fellowship
- NIHR fellowships
We are keen to engage with talented and highly motivated students, postdocs, and clinical fellows. If you are interested in joining our dynamic team, please contact Dr Tamir Rashid for more information about available positions in our lab.
- Deniz Kent (CEO, Prolific Machines)
- Samuel Blackford (Consultant, Dark Horse Consulting)
- Joe Segal (Senior Scientist, Biomarin)
- Chaozheng Li (Associate Researcher, Milner Therapeutics Institute)
- John Ong (Academic Clinical Lecturer, Cambridge Stem Cell Institute)
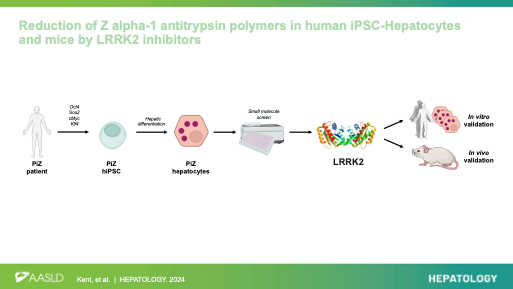
Reduction of Z alpha-1 antitrypsin polymers in human iPSC-hepatocytes and mice by LRRK2 inhibitors. Kent D*, Ng SS, * Syanda A*, …, Lomas D, Ebner D, Mueller C, Rashid ST. Hepatology 2024. https://doi.org/10.1097/HEP.0000000000000969
Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells.
Rashid T*, Corbineau S.*, Hannan N, Marciniak S, …, Lomas DA & Vallier L. Journal of Clinical Investigation 2010. https://doi.org/10.1172/JCI43122
Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells.
Rashid ST*, Yusa K*, Strick-Marchand H, Varela I, …, Lomas DA, Bradley A & Vallier L. Nature 2011. https://doi.org/10.1038/nature10424
Validation of cGMP-compliant human pluripotent stem cell-derived hepatocytes for cell-based therapy
Blackford, SJI, Ng, SS, Segal, JM, King, A, Kent, DH, Moore, J, Sheldon, M, Ilic, D, Dhawan, A, Mitry, R& Rashid, ST. Stem Cells Translational Medicine 2018. https://doi.org/10.1002/sctm.18-0084
Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold.
Ng SS, Saeb-Parsy, K, Segal, JM, Serra, MP, No, DY, Frank, CW, Cho, NJ, Nakauchi, H, Glenn, JS& Rashid, ST. Biomaterials 2018. https://doi.org/10.1016/j.biomaterials.2018.07.043
Single cell analysis of human foetal liver captures the transcriptional profile of hepatobiliary hybrid progenitors
Segal, JM, Kent, DH, Wesche, D, Ng, SS, Serra M, Teichmann S, Quake, S, *Nakauchi, H& *Rashid, ST. Nature Communications 2019. https://doi.org/10.1038/s41467-019-11266-x
Sulfated Alginate Reduces Pericapsular Fibrotic Overgrowth on Encapsulated cGMP-Compliant hPSC-Hepatocytes in Mice
Syanda, A.M, Kringstad, V.I, Blackford, S.J.I, Kjesbu, J.S, Ng, S.S, Ma, L, Xiao, F, Coron, A.E, Rokstad, A.M.A, Modi, S, et al*Rashid, ST. Front Bioeng Biotechnol 2021. https://doi.org/10.3389/fbioe.2021.816542
Our researchers
Dr. Tamir Rashid
/prod01/channel_3/media/images/people-list/T-Rashid.jpeg)
Dr. Tamir Rashid
Clinical Reader in Liver Regeneration
Adam Syanda
/prod01/channel_3/media/images/people-list/Adam-Syanda.jpg)
Adam Syanda
Research Assistant
Dr Soon Seng Ng
/prod01/channel_3/media/images/people-list/1602875743557.jpeg)
Dr Soon Seng Ng
Post-Doctoral Researcher
Elena Garitta
/prod01/channel_3/media/images/people-list/Elena-Garitta.jpg)
Elena Garitta
Visiting PhD Student (in collaboration with QMUL)
Zhuzhen Duan
/prod01/channel_3/media/images/people-list/Zhuzhen-Duan.jpg)
Zhuzhen Duan
Research Assistant
Li-An Brown
/prod01/channel_3/media/images/people-list/Li-An-Brown.jpeg)
Li-An Brown
PhD student
Ahmad Kheyami
/prod01/channel_3/media/images/people-list/Ahmad-Kheyami.jpg)
Ahmad Kheyami
PhD Student
Dongyang Li
/prod01/channel_3/media/images/people-list/Dongyang_Li%5B2%5D-copy.jpg)
Dongyang Li
PhD Student
Andrea Dominguez
/prod01/channel_3/media/images/people-list/Andrea-Dominguez.png)
Andrea Dominguez
PhD Student
Lowri Morris
/prod01/channel_3/media/images/people-list/people-list-placeholder-400X400px.jpg)
Lowri Morris
PhD Student
Vickie Wang
/prod01/channel_3/media/images/people-list/Vickie-Wang.png)
Vickie Wang
Placement student
Yu Ri Im
/prod01/channel_3/media/images/people-list/Yu-Ri-Im.jpeg)
Yu Ri Im
Honorary Clinical Fellow
Liang Ma
/prod01/channel_3/media/images/people-list/Liang-Ma.png)
Liang Ma
Honorary Research Associate
Fang Xiao
/prod01/channel_3/media/images/people-list/Fang-Xiao.png)
Fang Xiao
Honorary Research Associate