 In early 2014, Imperial PSTRC announced a call to Imperial College Healthcare NHS Trust staff and patients to submit applications for projects to improve patient safety and quality of care. More than 80 applications were received, shortlisted to 11 ideas that included healthcare apps, service improvement methodologies, point of care diagnostics and education and training. Finalists pitched their ideas to a panel of patient safety experts: Professor Ara Darzi; Yasmin Alibhai-Brown, journalist and author; Martin Bromiley, pilot and patient safety advocate; and Shona Maxwell, Chief of Staff, Office of the Medical Director, Imperial College Healthcare NHS Trust. The 9 winning projects received up to £30,000 in funding support and have been partnered with expert support and advice from PSTRC researchers to transform these ideas into innovative patient safety products or interventions within the Trust. Through this approach we have also raised awareness of our research portfolio amongst staff and supported Trust-wide engagement with patient safety improvement.
In early 2014, Imperial PSTRC announced a call to Imperial College Healthcare NHS Trust staff and patients to submit applications for projects to improve patient safety and quality of care. More than 80 applications were received, shortlisted to 11 ideas that included healthcare apps, service improvement methodologies, point of care diagnostics and education and training. Finalists pitched their ideas to a panel of patient safety experts: Professor Ara Darzi; Yasmin Alibhai-Brown, journalist and author; Martin Bromiley, pilot and patient safety advocate; and Shona Maxwell, Chief of Staff, Office of the Medical Director, Imperial College Healthcare NHS Trust. The 9 winning projects received up to £30,000 in funding support and have been partnered with expert support and advice from PSTRC researchers to transform these ideas into innovative patient safety products or interventions within the Trust. Through this approach we have also raised awareness of our research portfolio amongst staff and supported Trust-wide engagement with patient safety improvement.
Read press release: Imperial NHS Trust staff rise to the challenge to improve patient safety
Presentation by Dominic King, Karima Collins and Jeremy Bishop on Patient Safety Challenge projects from the 3rd Annual CPSSQ Symposium
Patient Safety Challenge Winners
- Using service improvement methodologies to reduce pressure ulcers
- Imperial App Surgical Site Infection Surveillance and Improvement (I-ASSISI)
- Optimising Responses to Emergency Situations in the Cardiac Catheterisation Laboratory: Using Team Based Training and Checklist
- Assessing and Reducing InSulin Errors (ARISE)
- The PSAQIM Project
- Automation of urinalysis at the Point of Care across ICHT
- Improving safety of inpatient anticoagulation and transfer of care
- Improving the safety of thickened liquids delivery in patients with oropharyngeal dysphagia and thin liquid aspiration
- Investigating the feasibility of training patients to identify adverse events
Project status: Completed. Final report submitted. Full report can be viewed here: A Review of Pressure Ulcer Data Analysis in Use
Background
The aim of the North West London pressure ulcer project has been to build up and expand the work carried out by the Community Education Provider Network (CEPN) team in 2013-2014. Imperial College Health Partners (ICHP) are engaging with various stakeholders to explore the benefits of a single joined up approach across our partners in NW London to improve the prevention and management of pressure ulcers.
The overarching aim of this project has been to align the existing pressure ulcer initiatives into a harmonised and collaborative project to achieve improved capability in preventing and managing pressure ulcers across the health care providers in NW London.
The project reported here was funded and supported by Imperial NIHR Patient Safety Translational Research Centre and focused on one particular aspect of pressure ulcer prevention and management: the development of a joined-up patient information system. However, the project team has been keen to learn and adopt best practice from other AHSNs and engage with subject matter experts nationally and internationally where this could be used to inform good practice in NW London.
2017 update
Outcome
Understanding the current diverse reporting shows there is a need for change if meaningful data is to be obtained. Susanne Coleman et al (2015) discussed findings of a national survey to compare PU monitoring systems in NHS inpatient facilities in England. The paper made five key recommendations for strategic change to reporting at national, regional and local levels.
Nationally, a decision should be made as to why reporting is required. Is it to ensure best practice or to castigate services for poor care? Streamlining national reporting systems need not mean poor care will ensue. Rationalising reporting structures. i.e. ST, NRLS and STEIS as well as the CQC, CCG and social services may be recommended as reporting the same ulcer to many organisations allows for duplication and adds to the burden of time-consuming paperwork. The findings from organisations in North West London reflect and support the findings of Susanne Coleman’s team.
Diagram 2 shows the plethora of different reporting pathways that currently exist.
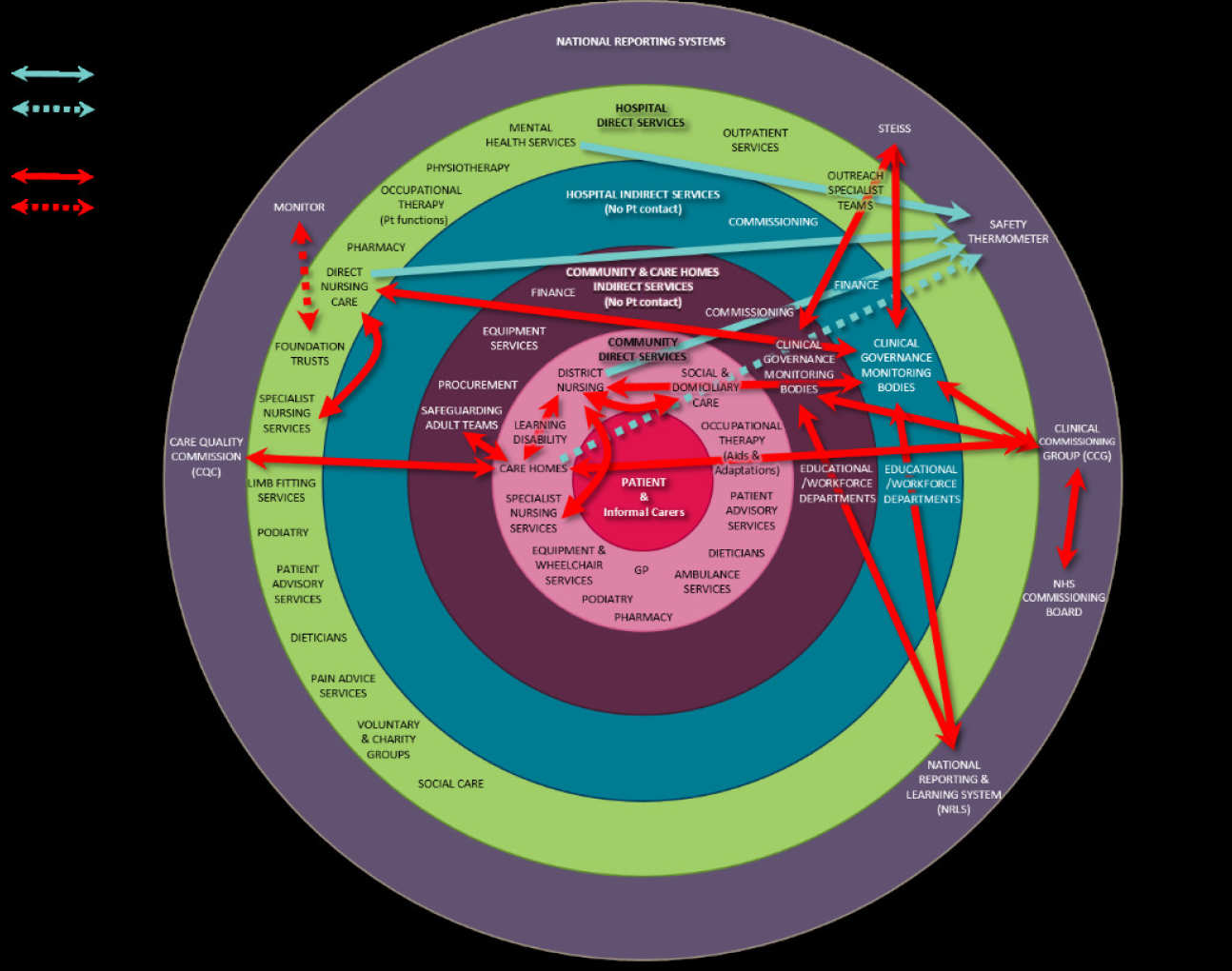
This diagram depicts the patient and carer at its centre
The pale pink – Community funded direct services
Purple - Indirect support services for the community
Blue – Indirect hospital services
The green circle – Direct hospital funded services
The outer purple circle – National reporting systems
Recommendations for Change in North West London
It is likely that national reporting structures will evolve over time in response to findings such as those produced by Coleman et.al (2015) as they did after the Serious Incident Framework (2015) however, this should not prevent action in NW London. Standardising data collection to improve communication and understanding in N. W. London can be achieved to compliment and improve national guidance.
The recommendations are to:
- Appoint a specialist forum of multi-agency carers and professionals for agreeing standardised reporting and practice. The buy in to change current reporting practice from the Executive level in each provider organisation has to be established across the sector if the changes are to be meaningful.
- All organisations including NHS MDT, care homes and social services to agree to collaborative working for the benefit of patient care. This means addressing the issue of attribution to enable learning rather than allocation of blame.
- Standardise the minimum data set for reporting. If there is to be a sharing of data between organisations there are anomalies which will distort results.
- Standardisation of the PU grading system and definitions of what constitutes a PU is required. i.e. moisture lesions, diabetic foot ulcers, device related ulcers. There are organisations that are not using the up to date NPUAP/EPUAP (2014) reporting definitions. Also some do not report device related PU’s and moisture skin lesions are known to be counted as PUs’ through lack of education. Those who do separate moisture lesions from PU reporting are noticing a reduction in the numbers of PUs’ they report. It is not a National requirement to report moisture skin lesions. However the incidents of moisture skin damage may be high so reduced PU incidents may not be in recognition of improved care.
- Decide on the importance of data collected and what it will be used for. Is it to understand numbers or compare one organisation with another or improve the patient journey? The decision to streamline reporting will reflect the rationale for reporting incidence, incident or prevalence monitoring. There is a need to share patient information to produce population based incidence data to enable accurate benchmarking and comparisons of origin of ulcer and deterioration of ulcer.
- Agree where to store incident reports in all organisations to enable staff swift access when asked for information. To record identifiable patient details in each incident report is to be commended however there needs to be reliable access to the record to establish previous reporting together with SI reporting history if applicable.
- Standardise the RCA reporting tool. Currently the national tool has been adapted within organisations. This has reduced the ability to share reliable data to inform education and learning.
- Agree a process for positive learning from RCA data to share best practice and to remove negative blame culture.
- Introduce a competency framework in PU grading and reporting across all organisations.
- A line of communication between the individual organisations to be formally recognised and adhered to with standardised communication documents; to include a definition of acceptable waiting times from referral to assessment, equipment access, recognition of the carer expertise.
- Introduce a referral pathway for domiciliary carers and care home staff to access services when an individual is at risk or who has a PU. The pathway to include actions for non-registered workforce to implement immediately
- Produce an option appraisal of the different systems and approaches that could be adopted to produce a standardised population based data set. This could include:
- All versions of Datix systems in use (see appendix 2). The limitations for using Datix is the lack of availability for a central dashboard
- Other systems in use such as SystmOne and Last Word
- United Lincolnshire Hospitals has a 10-year experience of developing an effective monitoring tool Ref: The development & benefits of 10 years’ experience with an electronic monitoring tool (PUNT) in a UK hospital trust. EWMA Journal 2015. Vol 15.no2. In approximately eighteen months from now there will be a licenced pilot version of PUNT made available and the final application ready eighteen months after that. This will be a wireless system and the hub will be located in Leeds.
- NW London CLAHRC is developing a generic web based patient safety dashboard which it might be possible to adapt to create a standardised population based information system. This approach will enable an agreed minimal data set to be collected by all organisations. The system of choice should promote live incident reporting and allow for a central dashboard to enable organisations to track individual patients across the system of care and link individual organisations to an overall picture of PU incidence. There may be an opportunity in the future to develop another bespoke data collection system for all organisations to use.
- Develop a central dashboard where organisations download agreed data set. The availability of this option will be dependent on which system is chosen and who agrees to purchase the system and support the upkeep. With patient identifier information stored on the dashboard data protection will be a key requirement.
- Patient Safety Collaborative to work with care homes to introduce ST data collection if this is the system of choice
- Provide a CCG supported training programme for the non-registered workforce for PU prevention including collection of ST data if this is to be collected across the system in NW London.
- To identify the number of beds/community caseload one TVN is responsible for is to be recommended so the role is accessible to all services /patients across the sector. A standardised competency framework for the role and responsibilities of a TVN is also required and made available to all.
IMPACT
Currently, the financial implications plus the detriment to quality of life for individuals is not clear. If there is a requirement to understand the burden of care pressure ulcers create for patients, and in the care system as a whole then all services nationally and across the sector should be collecting and sharing meaningful pressure ulcer data. To achieve this, there need to be system changes. The recommendations from this project will be useful in making those systematic changes.
Project status: Completed. Final report not submitted.
Background
Surgical Site Infection (SSI) rates are increasingly seen as markers for quality of care. The Trust has recently developed a committee to oversee and embed a Trust-wide approach to Surgical Site Surveillance. The committee is an overarching group, which links the existing SSI work to Trust-wide quality improvement initiatives, Academic Health Science Centre research themes, and international collaborations with the Trusts' partnership work with the World Health Organisation and THET (Tropical Health and Education Trust) on patient safety.
It is anticipated that SSI rates will be linked to CQUINs in the future, therefore, it is critical that methods for data collection, guidelines and practice are standardised throughout the Trust. While data is already being collected for neurosurgery, orthopedics and cardiac surgery to submit to Public Health, England, there is currently no data being collected for any of the other specialties.
2017 Update
Outcome
An app has been developed for SSI prevention guidelines. The I-ASSISI app has not been trialled in a clinical setting due to the need for it to be approved by the Trust App Committee.
Project status: Completed. Final report submitted. Full report can be viewed here: Optimising responses to emergency situations in the Cardiac Catheterisation Lab
Background
The number, range, and complexity of interventions performed in the cardiac catheterisation laboratory (CCL) are increasing rapidly. However, there are currently no standardised safety protocols for the CCL setting and so the risk of Patient Safety Incidents (PSIs) is high. How effectively a team responds to an emergency will often determine a patient’s likelihood of survival and the impact of acute treatment.
This may explain why research into patient safety incidents (PSIs) has shown that - rather than technical errors - it is failures of leadership, teamwork, and communication that are most commonly the root cause of errors. In many other industries and services (e.g. aviation/ the military/ nuclear power/ construction) standardised safety procedures are followed as routine.
Simulation training has shown to be very powerful in changing learned behaviours (reference 5, p284, ‘Principles of Team training’). Using modern software and equipment, healthcare staff can be exposed to a highly realistic emergency scenario which they are asked to respond to in real time. Subsequently, video analysis is used to provide constructive feedback and analyse both individual and team responses to emergencies. In the setting of the surgical operating theatre, this has been shown to be of benefit (GAWANDE NEJM paper), but this has not previously been attempted in cardiology.
2017 Update
Outcome
Six training sessions have been run since March 2015, covering cardiology complications in interventional, paediatric and electrophysiology. Since then, training has continued on a monthly basis. Training has included two types of scenario:
- Multiple complications, to develop teams’ ability to manage complex, fast paced emergencies, including following processes, such as the emergency blood protocol.
- Slower paced scenarios, such as anaphylaxis, which included periods of teaching, to help staff understand the processes and roles/priorities of staff called to assist (e.g. anaesthetists)
82 staff members have attended training (several more than once).
impact
The NIHR Imperial PSTRC funding has allowed the Trust to purchase the equipment needed to deliver simulation training indefinitely and the project manager has ensured that the training now has a dedicated slot, for which service managers release staff and there is a committed pool of consultant cardiologists who provide medical input. This means that training will continue indefinitely, with the ambition to train all cath lab staff at least once every year.
Project status: Completed. Final report not submitted.
Background
The prevalence of diabetes in in-patients in Imperial College Healthcare NHS Trust in the 2013 National Diabetes Inpatient Audit was up to 28.3% and consistently increased annually across all sites. Over the same period, prescription errors for people with diabetes have fallen but medication errors, management errors and insulin errors have remained the same or increased. A National Patient Safety Agency report found 972 incidents reported moderate harm and 18 had fatal or severe outcomes from insulin prescribing errors between 2003 and 2009 and diabetes prescribing errors are associated with hypoglycaemia and hyperglycaemia which contribute to increased length of stay, delayed wound healing and risk of secondary complications for in-patients with diabetes.
We propose a project led by a diabetes specialist pharmacist with aim of reducing diabetes treatment errors in the hospital. This will be achieved through an education programme for patients, prescribers and nurses, by process mapping insulin administration and be empowering self-administration and through the adoption of technology, incorporating insulin safety into Trust smartphone apps and preparing e-prescribing bundles to ensure the improved safety of medicines for people with diabetes.
Changes to medication, management and insulin error rates will be measured by repeat audit using the validated National tool.
2017 Update
Outcome
Milestones, all of which contribute to insulin safety across the Trust, to date are:
- Insulin needle policy agreed and procurement rationalised
- Education package to support needle changes finalised
- Insulin passport materials were rewritten and submitted to Drugs and Therapeutics committee for approval
- Clinical pathway for insulin-treated people in the hospital written and in consultation phase prior to Trust-wide launch and education.
Project status: Completed. Final report submitted. Full report can be viewed here: Development of web-based Patient Safety & Quality Improvement Meta data (PSAQIM)
Background
A wealth of information systems containing potentially useful information for patient safety and quality improvement exist within Imperial College Healthcare NHS Trust (ICHT). These include the infection control monitoring system and the pharmacy medication dispensing and stock maintenance system among many others. However, information about these systems, what data they contain and how they can be accessed is limited and typically only known by regular users. A trust Information asset register (IAR) is available but it requires the user to have knowledge of what systems currently exist rather than allowing users with no prior knowledge to find out what information exists and where/how it may be accessed to support review and monitoring of patient care and quality improvement. This presents a potential missed opportunity to improve frontline staff awareness of the data sources available in the trust that could support a more effective secondary use of existing data. In addition, healthcare trusts invest heavily in information systems and databases to support routine operations, yet the potential for effective re-use of existing information assets to support healthcare professionals in enhancing patient safety and quality of care is yet to be realised.
A meta-data prototype that provides information about the existing data available and enforces a synonymous process for all healthcare professionals to follow may be a potentially cost effective process in enhancing reuse of data to support quality and safety of patient care. A meta-data resource could potentially address the issue around users’ lack of data knowledge. “Meta-data” refers to the descriptive and practical information about a dataset, rather than the actual data itself. This type of tool helps provide stakeholders with the relevant information around data that exists and an integrated tool could be a good starting point for getting the answers to questions around access, content and potential applications of relevant datasets.
Each secondary care organisation varies in processes, structure, and services; hence the potential factors influencing the secondary use of data may differ too. Therefore, it is important to identify the barriers users are facing in trying to identify, access, and reuse existing electronic data in the NHS Trust within which the Patient Safety and Quality Improvement and Meta-data (PSAQIM) tool will be implemented. Information regarding the data that exists within the organisation and the reasons for which data is being reused by healthcare professionals and researchers should be identified, in order to produce a tool that presents information about existing electronic databases within the organisation concerned. The information users require in order to reuse data should be explored in order to answer the questions they have and present them with the information they require in a simple manner. However, from the users’ perspective, the process of granting access should be made simple and easy (a role that is commonly dealt with by the ICT team.
Therefore, qualitative interviews and a proof of concept study were conducted in the present study. The former explored the barriers and facilitators for SUD, and requirements for the meta-data tool from the perspectives of healthcare professionals and researchers who wish to reuse data. The latter then included development and evaluation of a meta-data prototype based on the user requirements identified through the qualitative interviews.
2017 Update
Outcome
The tool was produced based on the results of the deductive analysis, user proformas, and user specification; the PSAQIM tool was successful in addressing the barriers and limitations to the secondary use of data.
The study has had the following outputs:
- The production of functional requirements specifications with practical user cases embedded within a large qualitative interview study.
- Production and small scale evaluation of the meta-data prototype.
- Contributions to a larger study (i.e. an improvement science PhD) focusing on ways to enhance the secondary use of data in secondary care.
- The ICT team using this project as a pilot and developing a business case for the production of a meta-data tool.
- Project publications.
Impact
This study illustrates that a meta-data tool sign posting existing systems with a clear process for data access, and guidance for data reuse could help enhance the secondary use of data and address various factors that influence the secondary use of data. This can then assist in identifying practical solutions that may address not just one, but multiple limiting factors hindering the secondary use of data that exist in their organisation.
Project status: Completed. Final report submitted. Full report can be viewed here: Automation of Urinalysis at the Point of Care across ICHT
Background
POCT urine chemistry analysis is currently being done manually (dipstick method) across the majority of clinical areas requiring this service at Imperial College Healthcare NHS Trust.
The dipstick method is based on colour development and it relies on the ability of the user to accurately interpret the colour type and intensity. This method is subjective and open to interpretational error. Test results are transcribed into patient notes with potential for transcription error. No quality assurance procedures such as Internal Quality Control or External Quality Assurance (IQC or EQA) are in place and it is difficult to prevent use by untrained staff. There is significant potential for erroneous results and inappropriate/delayed patient management (e.g. missed cases of urinary tract infection [UTI]).
It is anticipated that automating the processes from testing through to reporting will significantly minimise the risks to patient safety whilst safeguarding staff and the Trust (in cases of litigation or patient queries). The automated readers have a QC and operator lock-out facility ensuring only trained staff can use the device and appropriate QC checks are carried out prior to the patient test. Test results will automatically transmit to electronic patient records – saving staff’s time and eliminating possible transcription errors. Results will be readily accessible and traceable with a full audit trail.
The safety and governance measures associated with the fully automated systems will enable the Trust to demonstrate compliance with relevant quality standards such as MHRA/UKAS and achieve accreditation for the service.
This project involves piloting automated readers and assessing the benefits over the manual dipstick method, with the hope of rolling urinalysis automation to the rest of the Trust.
2017 update
Outcome
The automated methods performed significantly better than the manual method in all areas assessed (data quality, governance, cost and, efficiency). However, the fully automated system demonstrated more benefits over the semi-automated method (stand-alone analyser) specifically by:
- eliminating transcription error
- ensuring appropriate quality control checks are carried out and passed, prior to device use
- allowing for automatic logging and storage of reagent information whilst also ensuring the integrity of reagents used (i.e. ensuring only in-date reagents can be used on the device)
- ensuring all results (patients, IQC, EQA) are appropriately recorded with no loss of data
- ensuring operators are correctly recorded with the required level of detail to allow for traceability
- significantly reducing the amount of time spent per sample with potential cost savings
It is worth mentioning though that the fully automated system relies on active IT links and there were times during the course of this pilot project that results were not transmitted across to the patient electronic records due to IT connection issues. However, the data remained on the analyser and was also permanently stored on the instrument data manager. In addition, the analysers provided result print-outs for immediate patient care, therefore, no negative impact on patients.
Impact
Use of the fully automated system will enable the Trust to provide a safer and more efficient POCT urinalysis service than currently, as well as meet relevant accreditation standards. The fully automated method has QC and operator lock-out facilities which can be centrally and remotely managed by the Pathology POCT team to ensure analysers can only be used by competent staff and only when appropriate QC checks have been done. A fully automated system will ensure consistency, standardisation (e.g. of units) and improved level of accuracy (than currently) whilst significantly saving on staff time.
Based on the findings from this study, we would recommend the use of fully automated system over the manual or semi-automated methods. The required IT links for the analysers have been put in place as a result of this pilot project so any new analyser can easily be connected to the hospital information system. There is, however, a need to test the capability of the established IT links in handling a larger roll-out especially as we have previously identified some issues with data transmission to Cerner.
The implementation of fully automated testing will have capital and ongoing cost implications and this should be considered prior to implementation.
Project status: Project did not go to completion due to staff turnover
Background
Warfarin is a widely recognised high-risk drug that poses a number of challenges including unpredictable patient response, numerous drug interactions, requirement for International Normalised Ratio (INR) monitoring in the inpatient setting which needs to be arranged, and continued once patients are discharged, and a slow onset and offset of action (not exhaustive). High INRs (>5.0) regularly contribute to increased patient stays costing the Trust money. High INRs can be due to inappropriate dosing of warfarin, lack of INR monitoring in the inpatient setting or lack of adequate follow up arranged for patient post-discharge (resulting in high INR re-admissions).
The aim of this project is to introduce three interventions to reduce high INRs, thus improving the patient experience and reducing the length of stay.
Project status: Completed. Final report not submitted.
Background
Thickened fluids are commonly prescribed to reduce the risk of aspiration and enhance swallowing safety in dysphagic patients who have difficulty controlling and managing thin fluids. Inconsistency in the labelling, definition, preparation and delivery of thickened fluids by healthcare workers increases the risks of patients experiencing unnecessary and avoidable choking, aspiration pneumonia and dehydration.
Currently, there is no training programme or standardised education tools for thickening liquids available for nurses or healthcare assistants. This contributes to great variability and frequent errors in the day-to-day preparation and delivery of thickened liquids.
The development of a training and education package with simple clinical tools for frontline carers in achieving the correct thickened liquid consistency is a long overdue patient safety intervention. Training and education will ensure that dysphagic patients always have access to liquids of the safest consistency as well as helping to contribute towards daily oral fluid intake requirements.
2017 Update
Outcome
The team developed professional posters, pocket-sized reference cards, stickers for thickener tin lids and an information booklet for patients and carers, which were printed for use. A simplified version of the app was also developed, which was accepted for Android and available for download.
View Brochure thickening liquids
View Information Booklet Thickening Liquids
View Poster thickening liquids
Impact
Implementation plan for the roll-out of resources to ward areas is in place. This will include displaying posters in clinical areas across hospital sites, issuing of pocket-sized reference cards to staff, and adoption of a flow process that ensures stickers are placed on thickener tin lids at the point of administration. Staff feedback on the quality, readability and usefulness of the clinical resources will be captured as part of resource roll-out and used to modify resources for the future.
The patient information booklet for patients and carers and the Android version of the smartphone app can be implemented immediately to support understanding, compliance and correct preparation of thickened liquids both in the short-term acute hospital setting and long-term community settings where appropriate.
Staff feedback on clinical resources will provide evidence on the impact of the training solution on improving knowledge, compliance and skills in the correct preparation of thickened liquids for patients.
Clinical resources will be integrated into the existing ward-based dysphagia training program for nurses as well as dysphagia awareness training for newly qualified nurses and allied health professionals.
Project status: Completed. Final report not submitted.
Background
Patient involvement is a key aspect of engagement in health-promoting behaviour and encouraging safe clinical care. A recent report by Don Berwick suggests that patient safety improves when patients evaluate their own care. It recommends that patients should be advising leaders on how patient safety can be improved. Since this report and that by Bruce Keogh, there has been a broad recognition that patients can make a meaningful contribution to improving patient safety in healthcare.
Patients have been involved previously in reporting medical errors and other adverse events during their healthcare experience and are often the only observers of the whole care journey and therefore will provide a unique perspective.
2017 UPDATE
Outcome
Sastrugi Software has been appointed to work on the project have produced an application based on current gold-standard data collection apps. The app is called EPIC: Empowering Patients to Improve Care. Initial drafts of the application received good feedback and a version was tested with patient groups.
During the initial phase of patient reviews, we recruited for structured focus groups and these were conducted towards the end of 2015. The patient involvement at this stage was crucial in shaping the latter stages of the project and what we hoped to achieve. Following qualitative analysis we have been through a number of iterations of the app design:
- Re-wording and re-ordering adverse events and errors
- App functionality including search options, layout, and example text
- The addition of an extra “positive option” from the homepage
Patients were very keen to be able to also report on positive aspects of their care, which has extended the scope of our project. We have also learned that far from being a potential burden there is a real passion for involvement and the potential added benefit of improving the patient’s experience simply through being involved.
EPIC is completed and ready to be used as a web-based app. It can be saved to the participants’ home screen and once logged in, it can be used offline. It only then needs to be online again when the patient is discharged and the data has to be uploaded.
(The link to the app is: https://epicpatients.com/.It can be accessed with the login: TESTUSER1)
Full ethical approval has been granted for the study so that recruitment can begin. General surgical patients are being recruited to use the app and record adverse events and errors in their own care. Uptake has been initially slow and the team is recording the reasons patients have for declining to participate, as this will form an important finding of the study.
The project team completed the initial round of recruitment for the feasibility in April 2017.
Impact
This is the first study to use technology to empower patients to take an active role in safety monitoring. By doing so we will have access to patient-specific data and the associated outcomes which will mean we can comment on or rank the societal and potential economic costs of AE.
Once we have demonstrated its use in identifying adverse events and allowing the rapid collection of QoL data we can use it to assess the impact of any new safety intervention. As this will be occurring rapidly, in near real time, we will be able to facilitate the rapid comparison of exiting and novel patient safety interventions.
By expanding the study to follow up patients in the community we can further understand the events affecting our patients post discharge and what is important to them. This is a poorly understood area given the difficulties of data capture in this setting.

