When developing new therapies, drug molecules must be combined in a formulation with other ingredients that help them reach the disease target while minimising harm to the patient. An IMSE team of material scientists, chemical and mechanical engineers, biologists and clinicians is developing new ways to accelerate the design of effective drug formulations.
Synthetic polymers are used because they can be tailored to specific diseases and organs by tuning their chemistry. The team is developing new algorithms to identify the most promising polymer formulations from hundreds of millions of options and is finding new ways to make precision polymers and test their efficacy quickly. They are applying this approach to diseases of the central nervous systems (brain cancers and Parkinson’s disease), where a key challenge is for drugs to cross the blood-brain barrier.
Developing effective treatments against disease requires much more than a drug molecule that can target a specific type of cell or protein. It is often extremely difficult to get a sufficient amount of drug to reach the disease target due to the body’s natural barriers. For example, the gut can prevent oral drugs from reaching the bloodstream, while blood-brain barrier and blood-eye barrier, among others, can prevent access to specific organs. Simply upping the concentration of drug in the body is not effective as it can lead to severe side effects as the drug reaches healthy cells. Indeed, ensuring that drugs only reach target cells is a key challenge in developing new therapies.
Drug delivery systems (DDSs) have emerged as an essential tool to enhance the efficacy of treatments, enabling drug molecules to reach the target disease site with limited detrimental impacts. DDSs come in many forms including devices, e.g., microneedles, and formulations, e.g., liposomes – vesicles made from lipid bilayers that can encapsulate the drug. Polymersomes (vesicles made from polymers) and polymer gelsare an especially promising class of formulations. Because they are based on synthetic polymers, their properties are highly tunable: vesicle size, response to temperature changes, drug solubility, surface charge and polarity can all be optimised to the specific disease and drug molecule. This opens the way for the development of much more effective therapies and even the repurposing of existing drugs that have previously been unable to cross some of the body’s barriers.
Despite the promise offered by polymer-based formulations, the process of developing an effective therapy tuned to the disease and drug remains slow and challenging. First, it is not easy to choose a “good” formulation given the vast chemical space of possibilities, with many building blocks (monomers) on offer to build the polymers and other choices such as the number of monomers in each polymer, the solvent used to form the gel/polymersome, the choice of ligands and other surface modifications – all decisions that have a big impact on efficacy. Second, once a formulation has been chosen, testing its efficacy is a long and expensive process because many difficult experiments must be carried out to make the formulation, determine its physical and mechanical properties, assess its performance in vitroand finally in vivo. Designing a DDS is like searching for a needle in haystack.
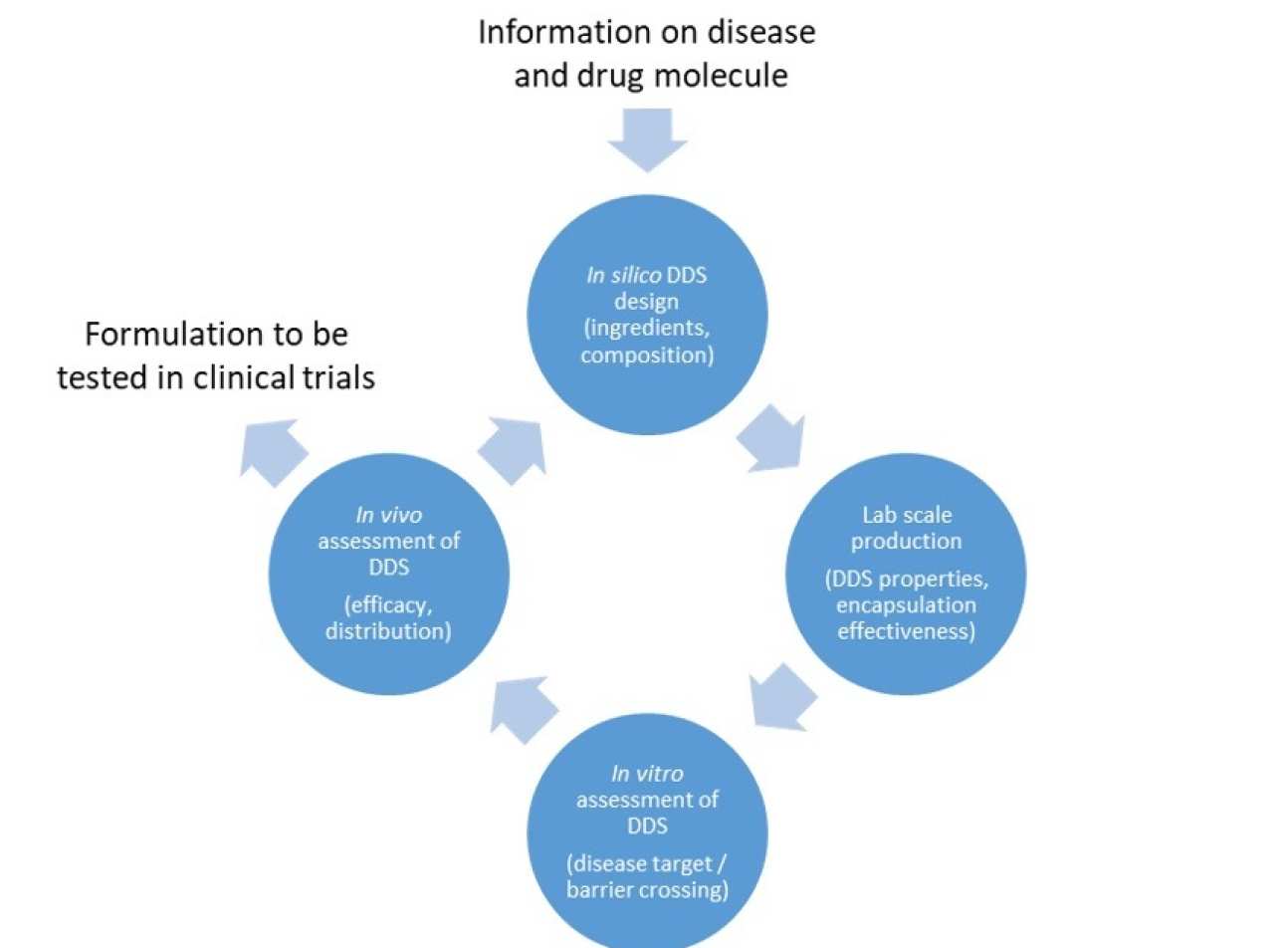
A multidisciplinary team of IMSE researchers is developing a novel strategy to accelerate the discovery of efficacious formulations (see Figure). Uniquely, it starts with the identification of promising DDS formulations in silico, using an approach that builds on all existing knowledge of what makes a good formulation, combining mechanistic understanding with the cautious use of rules-of-thumb and using state-of-the-art algorithms to identify promising DDS formulations amongst hundreds of millions of possibilities. This leads to a prioritised list of experiments that can be undertaken to develop an effective formulation.
At the moment we have made diblock copolymers, both AB and ABA and we are varying the chemistry and the block ratio to see how this affect the polymersome formulation and size. We also started some preliminary results with the structures that have formulated polymersomes to investigate how their encapsulation efficiency. Preliminary in vitro experiments to evaluate their toxicity are also been carried out. Also, the attachment of imaging agents that will allow us to monitor these vehicles in vitro and in vivo is underway.
At each stage of the process, new understanding can emerge and this can be embedded in the in silicodesign step and used to revise the prioritised list of experiments, continually accelerating the discovery process.
The research team is focusing on diseases of the central nervous system such as brain tumours and Parkinson’s disease, where the blood-brain barrier makes it especially difficult for drugs to reach disease sites.
