The work of Professor Peter Kohl's group
Peter Kohl heads up the Cardiac Biophysics and Systems Biology group at Imperial which tackles heart science issues at various scales.
Perhaps more than any other organ, the heart has fascinated thinkers and scientists through the millennia. Indeed, the Greek philosopher Aristotle, who studied the organ forming in chick embryos, concluded that it was the seat of intelligence, motion, and sensation.
Of course we now know that consciousness arises in the brain; nevertheless the allure of the heart remains strong in the public’s imagination, while research continues apace.
For Professor Peter Kohl (National Heart & Lung Institute) – head of the Cardiac Biophysics and Systems Biology group at Imperial – part of the mystique lies in the fact that the heart is self-regulating and propagates its own electrical activity.

Professor Peter Kohl
“It’s an absolutely magical organ; the fact that the heart can continue to beat once taken out of the body, for example during transplantation, is quite amazing really,” he says. “Of course, we can explain this, but I think it’s fair to be thoroughly impressed by the functionality of an organ that has the ability to beat, once every second or so, for 60, 70, 80 years. If it stops, life ends. It cannot afford to have the same number of breakdowns as a car has.”
This reliability is possible because of built-in feedback and feedforward mechanisms, which involve cross-talk between the electrical and mechanical aspects of the heart.
“If you increase blood return to the heart – for example when you sit or lie down – the myocardial muscle senses that, and the very next beat will be stronger. That is an observation from the 19th Century, yet we are still exploring the detailed mechanisms that underpin it.”
Peter’s group is a multidisciplinary one with a diverse array of research projects incorporating aspects of clinical medicine, systems biology, physics and engineering.
The fact that the heart can continue to beat once taken out of the body, for example during transplantation, is quite amazing really
– Professor Peter Kohl
“One of the things we like to do is to study behaviour across multiple spatial scales, from isolated cells to tissue and organ levels. The challenge lies in trying to relate what we learn from say studying a single ion channel in a cell to an ECG recording in a patient – it is not a trivial task.”
This undertaking is aided by a range of state of the art tools such as computer modelling of electrical and mechanical cardiac activity, as well as optical imaging of heart tissue using fluorescent ‘reporter’ dyes.
“If you use suitable camera systems you can track the wave of electrical signals as it spreads through the organ. Essentially – you make electrics visible, something I still find really amazing. We are very thankful to our colleagues who investigate stars because it’s their specialist cameras that are now being applied to biological systems!”
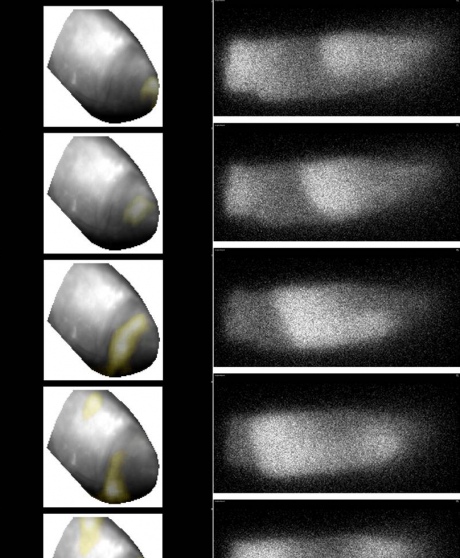
Left: spiral wave of electrical excitation at the apex of the heart, covering a tissue volume containing some 12 million cells, visualised using fluorescent reporter. Right: spiral wave of calcium release in a single cardiac myocytevisualised using a Ca2 -sensing flurescent dye.
One of the key insights to have emerged from these studies is the importance of the network of supporting cells that assist the function of myocytes (muscle cells). This includes nerve cells, smooth muscle cells, and connective tissue cells called fibroblasts – which Peter’s team is particularly interested in.
“It’s sobering to realise that there are more fibroblasts in the heart than myocytes; they are small and don’t contract beat by beat, but as ‘signalling hubs’ they are very important for the development and normal functioning of the heart. This relevance extends well beyond their primary function to produce the deformable skeleton that allows myocyte forces to be translated into pumping action.”
Indeed, recent findings raise the possibility that fibroblasts have an active role to play in the electrical activity of the heart.
“If we are looking at a voltage signal, we assume that signal comes entirely from the myocytes – but that’s not necessarily the case.”
In particular fibroblasts are generating a lot of interest in the context of scars. This includes scars that are caused by myocardial infarction (heart attacks), or applied as a therapeutic measure in patients with pre-existing atrial fibrillation (AF).
AF is a heart rhythm disturbance (collectively known as arrhythmias) which causes the atria to cease pumping. This reduces cardiac performance and carries the risk of blood clot formation. A standard treatment for this condition is catheter ablation, where some of the atrial tissue involved in propagating the rogue rhythm is essentially burned away, leaving a scar. However, in up to two-thirds of patients who have such ablation, electrical activity in the burned tissue makes a comeback, and the electrical impulse starts to propagate through the scar tissue again.
“If we could only make scars left after a heart attack slightly less dangerous and scary than they are now – that would be a good thing!”
– Professor Peter Kohl
“One possible explanation is that the connective tissue in these scars starts to act as a conductor. That was previously seen as unthinkable by many in the field, but the evidence in favour is starting to look quite compelling.”
Meanwhile, in patients who have survived a myocardial infarction, the resultant scar tissue can give rise to another type of disruptive rhythm, called a ventricular tachycardia. Here the danger lies in the fact that the scar tissue can regionally delay the electrical wave, compared with the main electrical propagation, and set up a second wave that distorts the activity of the heart, often with lethal consequences.
“I would like to explore whether we could make ‘better scars’ for patients through targeted changes to the electrical integration of connective tissue cells with myocytes. This might allow us, on the one hand, to improve the efficiency of atrial ablation lines so they that don’t re-grow connections, and on the other hand, to make infarcts in the main heart chambers electrically invisible.”
Ultimately, this would improve current therapies for atrial fibrillation, and reduce at least some of the dangers associated with scars left after a heart attack. While Peter acknowledges that this might seem like a modest aim, compared with regenerative and stem cell research directed at growing new muscle, he is convinced that it could bring benefit to a great many patients.
“If we could only make scars left after a heart attack slightly less dangerous and scary than they are now – that would be a good thing!”
Peter was recently awarded €2.5m from the European Research Council’s Advanced Grants fund, while other projects in the group are supported by the British Heart Foundation, Research Councils UK, the European Commission and the Magdi Yacoub Institute.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Andrew Czyzewski
Communications Division