Why snails don't wear sunscreen

Photograph: G.-U. Tolkiehn.
Researchers from Imperial College London develop new tool to track toxic waste products in the environment.
Paint, batteries, kitchen cleaner, medicine… These products have become part of our daily lives: We consume them, and after we are done, we dispose of them in the bin or down the drain. By doing so we produce a lot of waste, which eventually makes its way into the natural environment, seeping through soils and flowing through rivers and towards lakes, estuaries and seas. If present in high enough concentrations, this waste can be harmful to fauna and flora, contaminating their natural habitats and entering their diets. This is worrying from an ecological perspective, but also from a medical one, as organisms enter the food chain and eventually end up on our plates.

Contained in many of these common products are what we call nanomaterials. Nanomaterials are tiny feats of engineering, no larger than a few millionths of a millimetre in size. That’s about ten thousand times thinner than your average string of hair. But despite their minuscule size, nanomaterials achieve great things. They are used in everything from healthcare to electronics.
Within the nanomaterial family, zinc oxide nanoparticles, nano-sized particles made of thousands of atoms of metallic zinc and oxygen, are particularly important. Zinc oxide is a mainly insoluble, white powder commonly used as an additive in products such as plastics and adhesives. As a nanoparticle, it is used in paint, coatings, and, more importantly, in many sunscreens and cosmetics.
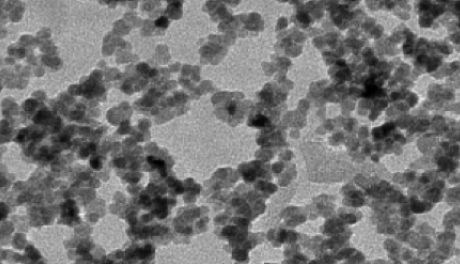
Zinc oxide nanoparticles under the microscope
While it is harmless in small amounts, zinc in higher concentrations can be toxic, and concerns have been raised over whether zinc-based nanoparticles could be harmful to the environment. But because these particles are so small, it is difficult to fully understand how they behave in nature and what their impact might be on humans, plants and animals.
By artificially exposing water-living organisms such as algae and snails to zinc oxide nanoparticles, researchers have started to understand how these nanoparticles interact with the living world.
Once the nanoparticles make their way into water as waste, they behave in one of two ways: they will either cluster together to form larger particles, a process called aggregation (a bit like many small soap bubbles clustering into a bigger one), or simply dissolve in the water, as sugar does in your morning cup of tea. The bigger particles can also attach themselves to the snails’ food and get swallowed whole by the organism.
 So in what way do the particles become toxic to natural organisms? Do the animals incorporate the particles that aggregate, or those that dissolve? It is important for scientists and manufacturers to know this so that products can be better designed and controlled to avoid harmful side effects.
So in what way do the particles become toxic to natural organisms? Do the animals incorporate the particles that aggregate, or those that dissolve? It is important for scientists and manufacturers to know this so that products can be better designed and controlled to avoid harmful side effects.
The problem is that it’s very difficult to track zinc from a nanoparticle once it enters the water and a living organism. Zinc is an element that is present naturally in the environment, including in waters, animals and plants. So how, once an animal has incorporated zinc into its body, do we distinguish the potentially toxic zinc of the nanoparticles from the ‘natural’ one?
Until today, scientists have only been able to achieve this by artificially exposing organisms in the laboratory to nanoparticles in much higher concentrations than you would actually find in wastewater. There is then so much zinc that they can see a how the animal responds to it. While this has helped researchers understand the behaviour of nanoparticles in water and organisms, it does not provide a realistic polluted environment and does not allow them to communicate strong conclusions to manufacturers.
But scientists from the MAGIC group in the department of Earth Sciences and Engineering at Imperial College London have thought out a solution. They decided to use a slightly different zinc to make their tiny nanoparticles; a type of zinc that could be traceable in the water and in the animals swimming in it, even in relatively small concentrations.
Now an atom is made of three basic ingredients: electrons, protons and neutrons. Electrons and protons are a bit like the yin and the yang. They balance each other, so that an atom must have the same number of electrons and protons to be stable. Neutrons, on the other hand, are so-called because they are “neutral”. They contribute to an atom’s mass and structure but have no specific properties as such, so their number can vary without having an impact on the atom itself.
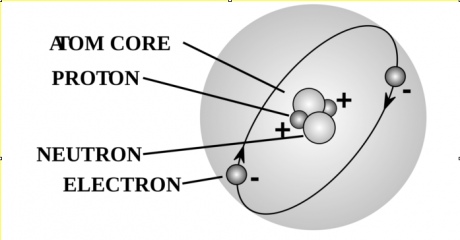
A natural atom of zinc has 30 protons and 30 electrons, but can have different numbers of neutrons. If you were to pick 100 atoms of zinc at random in nature, about half would have 34 neutrons, and the rest would have between 36 and 38. So the neutron composition of natural zinc is a mixture of all of the above ingredients.
Researchers Adam Laycock, Dr Fiona Larner and Prof Mark Rehkämper then thought: what if they artificially made nanoparticles with only one number of neutrons, rather than the natural mixture described? What if they made nanoparticles with, say, 100% atoms bearing 38 neutrons? That zinc would be different to natural zinc and would act like a fingerprint, traceable in the water and in the animals.
So the team re-created their very own natural environment in the laboratory. They designed a fake estuary and filled it with common water-dwelling snails. They then released their special zinc oxide nanoparticles in the water, exposing the snails to them and using the unique zinc as a tracker, following it from the water, into the snails and back out again.
The research team found that the zinc from the nanoparticle mainly entered the animal’s body in its dissolved form, rather than being swallowed with their food as a solid aggregate. These new results, published last month in the journal Environmental Science and Technology, will help nanoparticle manufacturing laboratories better understand their products and tailor them to prevent harmful effects to animals and humans.
Follow us on Twitter @mle_marion and @imperialrsm for more exciting research and Earth Science news!
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Marion Ferrat
Centre for Environmental Policy