Imperial researchers studying how blood vessels stay healthy
Drs Anna Randi and Graeme Birdsey are trying to understand how blood vessels form and repair themselves and the role they play in various diseases.
Since time immemorial humans have rightly linked blood flow to life and health – although this has often led to misguided interventions such as bloodletting and leeching.
We now have a quite comprehensive understanding of the role of the circulatory system in human physiology; but researchers are still trying to better understand how blood vessels form, how they are repaired after damage and the precise role they play in various conditions including heart disease, cancer and dementia.
Key to this field of vascular science are endothelial cells, which line the entire circulatory system – from the heart to the smallest capillaries – controlling what goes in and what goes out.
Double-edged sword
Two researchers who have dedicated their careers to understanding these very busy cells are Drs Anna Randi and Graeme Birdsey (National Heart and Lung Institute) who are based at the Imperial Centre for Translational and Experimental Medicine (ICTEM), which opened last year at the Hammersmith Campus.
“A lot of work over many decades has clearly identified these cells as key to maintaining healthy, happy and functioning blood vessels,” says Anna. “They form a single sheet, and they do this through cell-to cell-signalling; we still don’t know how they do it exactly, but it’s a beautiful mechanism.”
In cancer, this process of forming new blood vessels, which is essential for life, goes into overdrive as new blood vessels are sprouted to support the growing tumour. A whole class of new drugs is currently being developed that inhibits blood vessel formation (known as angiogenesis) and stymies the sustaining blood flow to the tumour, killing it off.
Endothelial cells are key to maintaining healthy, happy and functioning blood vessels
– Dr Anna Randi
By contrast, in the case of cardiovascular disease, endothelial cells become dysfunctional because of smoking, high blood pressure and elevated levels of low-density lipoprotein (LDL) cholesterol. Eventually this can lead to the formation of atherosclerotic plaques, which can narrow the arteries with potentially serious consequences such as myocardial infarction and chronic heart disease. Therefore scientists are trying various ways of stimulating the formation of new vessels in place of the diseased ones, a strategy called therapeutic angiogenesis. This is already being tested on patients around the world.
Cultivating blood cells
Anna’s research group at Imperial works with cell cultures obtained from the blood vessels of human umbilical cords. Like their native counterparts, these endothelial cells form a single layer on a plastic dish.
“It’s exactly the same as what we see in a real blood vessel; it’s a good system to understand the biology of these cells,” Graeme says.
They use microscopy in combination with molecular techniques such as fluorescence antibody tagging and gene targeting to identify and track important signalling molecules.
One protein that has emerged as particularly interesting is ‘Erg’, which Graeme describes as a ‘master regulator’ of endothelial cell function. It belongs to a class of protein known as transcription factors, whose job it is to bind to sections of DNA, switching genes on and off and creating a mosaic of genetic expression that programmes the unique identity and function of endothelial cells.
This is evidenced by experiments where Graeme and Anna’s team blocked the action of Erg using so-called ‘antisense’ molecules – with profound consequences.
“If you take Erg away, the contacts between cells destabilise and you get leakiness – not what you want in blood vessels,” he says.
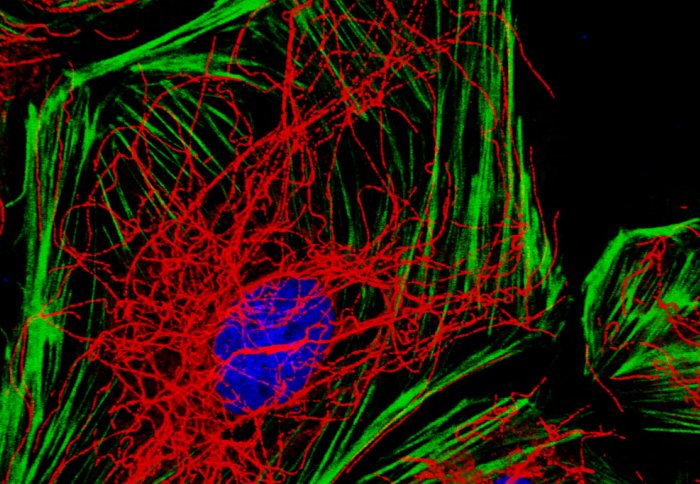
This is because Erg maintains the cells’ internal scaffolding, made up of actin fibres and a dense web of microtubules. This is demonstrated beautifully in the microscope image shown below, which was recently shortlisted for an image competition run by the British Heart Foundation.
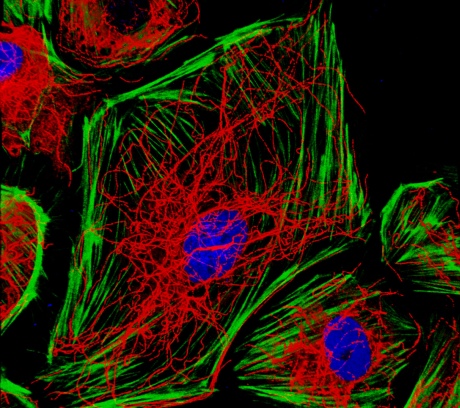
Endothelial cells with actin stress fibres in green and microtubules in red
“Images like this strike a chord with people who are non-scientists because they allow you to see what a cell really looks like; it’s not just a flat black and white picture in a book. It’s a very good tool for communicating what we do,” Graeme says.
Pulling together
Further experiments have shown that Erg also has a critical function in endothelial cell migration which is important for making new vessels as well as repairing them.
This was proved in an elegant series of experiments where Graeme prepared two separate dishes of endothelial cells – one with the Erg intact and the other with Erg blocked.
He made a cut straight down the middle of the cell sheet and watched them move using timelapse microscopy (see the video below). In the Erg-positive dish, the cells at the edge of the simulated wound migrated inward, eventually sealing the gap, whereas in the Erg-minus dish the cells simply dithered, seemingly not able to coordinate an effective response.
Genetic profiling of endothelial cells has shown that Erg controls a complex web of more than 2000 genes, themselves involved in migration, angiogenesis and endothelial cell function. The challenge will be to untangle this web and map the precise relationship between molecules. Hopefully this will identify some promising targets for treatment, which could for example, limit new blood vessel growth in cancer tissue, or promote the formation of new vessels in the heart after myocardial infarction.
Relevant References
GM. Birdsey, NH. Dryden, AV. Shah, R. Hannah, M Hall, DO. Haskard, M Parsons, JC. Mason, M Zvelebil, B Gottgens, AJ Ridley, AM. Randi. The transcription factor Erg regulates expression of histone deacetylase 6 and multiple pathways involved in endothelial cell migration and angiogenesis Blood. 119(3):894-903;2012
NH Dryden, A Sperone, RL Hannah, GM Birdsey, S Taufiq Khan, JA Layhadi, JC Mason, DO Haskard, B Göttgens, AM. Randi. The transcription factor Erg maintains endothelial quiescence by repressing NF-κB- dependent activity transcription through a novel ETS:NF-κB motif Journal Biological Chemistry, 287(15):12331-42;2012
Sperone A, Dryden NH, Birdsey GM, Madden L, Johns MJ, Evans PC, Mason JC, Boyle JJ, Haskard DO, Paleolog E, Randi AM. The transcription factor Erg inhibits ICAM-1 expression and vascular inflammation. Atherosclerosis, Thrombosis and Vascular Biology, 31:142-50;2011
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Andrew Czyzewski
Communications Division