An attractive solution to click chemistry
Research on catalytic nanoparticles carried out in the Department has recently been published in the leading journal, Chemical Communications.
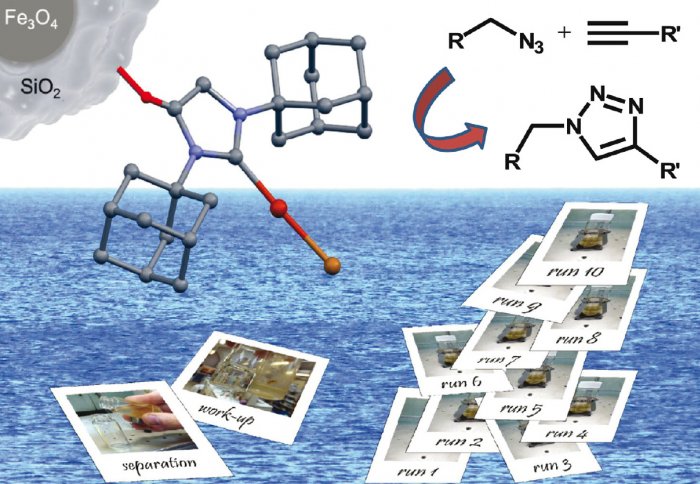
Research on catalytic nanoparticles carried out in the Department has recently been published in the leading journal, Chemical Communications as well as appearing on one of the covers. John-Michael Collinson and his supervisors, Dr Silvia Díez-González and Dr James Wilton-Ely, have developed a highly active catalytic system based on organometallic copper complexes immobilised on magnetic nanoparticles. This was applied to the reaction of azides and alkynes to form triazoles (often known as a ‘Click reaction’). Water was used as the solvent.
The term Click Chemistry refers to simple, selective, atom-economical, high yielding transformations using benign solvents (preferably water) which minimise waste and do not require purification by column chromatography. This approach has become a very important tool in the armoury of synthetic chemists but requires selective catalysts which can operate at low loadings. Normally the catalysts used for this reaction are removed from the product and discarded, frequently requiring additional purification steps. The principles of Click Chemistry are frequently adhered to rather selectively, especially regarding the choice of (often harmful) solvents and purification procedures.
The immobilised copper complexes reported in this communication are straightforward to form and show the same performance as the best known catalysts for this reaction. However, thanks to the iron oxide core of the support, they can also be separated using a hand-held magnet and recycled up to 10 times, minimising the use of solvents for purification and reducing copper waste. The article is open access and can be found here (Chem. Commun. 2013, 49, 11358).
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Maria Tortelli
Department of Chemistry