Magnetic molecular films are getting hot
Magnetism in a molecular thin film traditionally used in plastic electronics survives at temperatures reaching up to 100K.
This astonishing result was published today in Nature Communications by scientists from the London Centre for Nanotechnology (LCN), a joint venture between Imperial College London and University College London.
In their work, the team used flexible thin films of cobalt phthalocyanine (CoPc), a well-known industrial pigment and semiconductor. Those molecular semiconductors have advantages such as flexibility, low cost and ease of processing and are used in commercial applications such as organic light emitting devices and organic solar cells.
The magnetism of molecular materials is a recent discovery, but the phenomenon occurs at very low temperatures only reachable using complex and expensive cryogenics. In the new LCN study, magnetism is observed above the boiling point of liquid nitrogen (77K), a common and cheap refrigerant. This is particularly significant because it demonstrates that magnetism in these molecular films could eventually be exploited practically.
CoPc molecules have a magnetic property, called quantum mechanical "spin", that can be oriented in different directions, just like a compass needle. In a solid, the molecules stack on top of one another, causing the spins on neighbouring molecules to point in opposite directions. This produces a so-called anti-ferromagnet (see image).
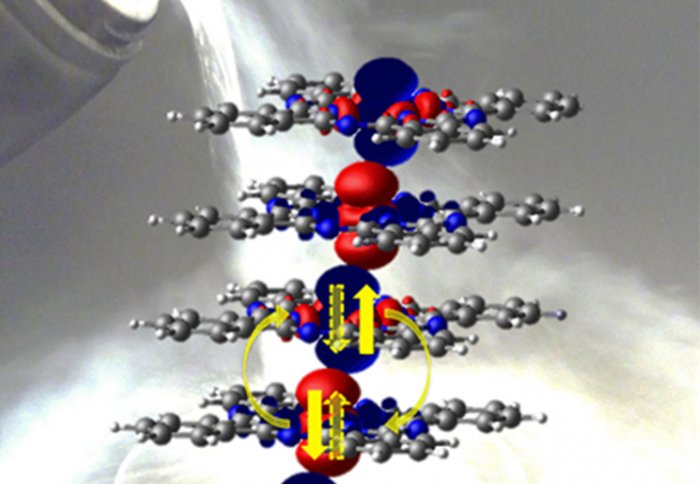
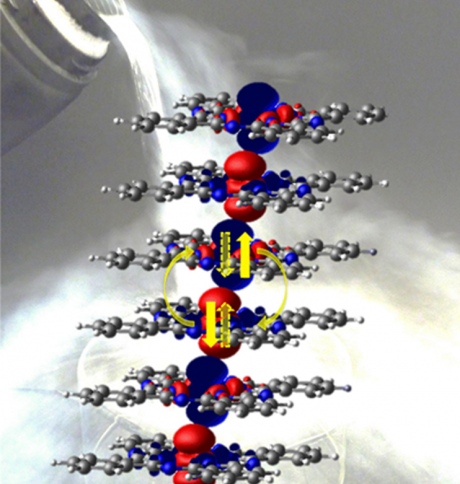 Distribution of spin orientations in a chain of six CoPc molecules, with blue and red representing spins pointing up or down respectively. The spins located on the Co atoms at the centre of the molecules couple anti-ferromagnetically up to temperatures above the boiling point of liquid nitrogen. The quantum entanglement follows because the spin pairs existing in a superposition of 'up-down' and 'down-up' states. The carbon, nitrogen and hydrogen atoms are depicted in grey, blue and white respectively, while the Co is at the centre of the molecule. Credit: Wei Wu, Gabriel Aeppli and Sandrine Heutz.
Distribution of spin orientations in a chain of six CoPc molecules, with blue and red representing spins pointing up or down respectively. The spins located on the Co atoms at the centre of the molecules couple anti-ferromagnetically up to temperatures above the boiling point of liquid nitrogen. The quantum entanglement follows because the spin pairs existing in a superposition of 'up-down' and 'down-up' states. The carbon, nitrogen and hydrogen atoms are depicted in grey, blue and white respectively, while the Co is at the centre of the molecule. Credit: Wei Wu, Gabriel Aeppli and Sandrine Heutz.
Michele Serri, the first author of the paper, explained that "for most molecular systems the strength of the interaction between spins is tiny, and a thermal energy of just a few degrees above absolute zero can disrupt the ordering.
"However, for CoPc films the interaction is much bigger and the ordering therefore can survive up to much higher temperatures. Excitingly we also see that the coupling can be suppressed if we slightly modify the structure of the crystal, so the material behaves like a magnetic switch."
The LCN scientists explain the experimental result using several theoretical techniques. According to LCN researcher Wei Wu, "the electrons in the molecule that are responsible for the magnetism spend much more time outside the molecular plane than within the plane, as depicted by the red and blue surfaces in the picture (see image). This greatly enhances the travel of electrons between the stacked molecules, resulting in a large interaction and hence a high magnetic transition temperature."
Remarkably, the theoretical calculations also reveal that these interactions could reach well above room temperature if the molecules could be arranged to stack directly above of one another. The predictions offer a new route for engineering room temperature magnetism in phthalocyanine-based materials.
The LCN discoveries create an important role for molecular materials in the emerging field of spintronics, which exploits not only the charge but also the spin of the electrons to develop more powerful and low energy information technologies.
In particular, the data in the paper indicate that the CoPc films contain spins in "quantum superposition" states, i.e. the compass needles are behaving as true quantum mechanical objects pointing North and South simultaneously. This implies potential utility for quantum computing.
The recently awarded UK Centres for Doctoral Training in Plastic Electronics and Theory and Simulation of Materials at Imperial College and Delivering Quantum Technologies at University College London will be particularly timely in facilitating the collaborative research underpinning exploitation of the team’s findings.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Press Office
Communications and Public Affairs
- Email: press.office@imperial.ac.uk
