NMR provides fresh atomic insights into a key protein that helps repair DNA
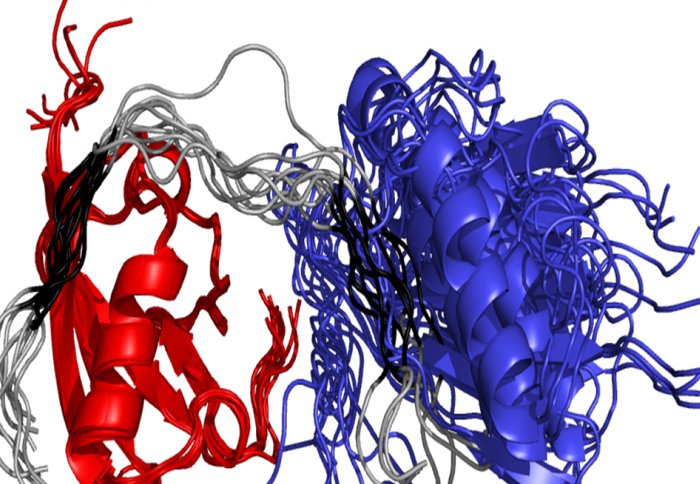
Image shows flexible RNF4, in grey, binding and manipulating a SUMO chain (blue and red)
Scientists have made an important advance in understanding how a protein that identifies and repairs cancerous cells operates.
RNF4 is a type of regulatory protein that controls the function and fate of proteins in cells by chemically flagging them. Proteins like RNF4 play an important role in many biological processes such as the response to DNA damage. Mismanagement of this is directly linked to cancer, and so researchers are exploring new insights into this process to help combat the disease.
Until now scientists have found in difficult to study how this protein works in detail, because it is partially disordered and can take on many different three-dimensional (3D) shapes.
In a new study published in Nature Communications, two teams of scientists from Imperial College London and the University of Dundee have succeeded in visualising this flexible protein for the first time, using Nuclear Magnetic Resonance (NMR) spectroscopy at Imperial’s Cross Faculty NMR Centre (CFNMR – http://www3.imperial.ac.uk/nmrcentre). The new images provide fresh insights into how RNF4 operates, and the scientists hope this new understanding could ultimately help improve cancer treatment.
Study author Professor Steve Matthews, from the Department of Life Sciences at Imperial College London, said: “Scientists have tended to ignore disordered parts of proteins because they have been too difficult to study structurally, but by using Nuclear Magnetic Resonance,(NMR) we are beginning to understand just how influential these proteins are in biology. We normally use X-ray crystallography to identify the molecular structure of proteins, but it’s unable to deal with flexible and fuzzy proteins like RNF4.
“The difference between what we can do with crystallography and NMR is like the distinction between taking photographs to appreciate something or recording a video. We can only guess how a bird flies when only shown pictures of it sitting in a tree or on the ground. X-ray crystallography is similar in that we visualise snapshots of proteins in different states. But with NMR we can follow how proteins alter their shape, much like capturing the bird flying on video, so we can now see all the steps in between,” added Professor Matthews.
Our cells are able to check whether their DNA has been damaged and are constantly making decisions on whether or not to make a repair. This decision-making process is controlled by an elaborate array of proteins. Many are involved in controlling DNA repair checkpoints. This can be done by chemically tagging target proteins with another protein called ubiquitin.
RNF4 uses a flexible tail to locate chains of another ubiquitinâlike protein, known as SUMO, and then adds the ubiquitin tag, signalling that this protein needs to be removed and destroyed. The cellular DNA repair machinery can then be accurately loaded and can proceed to the next stage. Failure of any of these steps can lead to cancer.
The new detailed molecular images reveal how the disordered part of RNF4 can recognise and deliver SUMO chains to the ubiquitin-attachment site, despite being inherently floppy.
REFERENCE: Xu, Y et al. ‘Structural insight into SUMO chain recognition and manipulation by the ubiquitin ligase RNF4’. Nature Communications. June 2014. DOI: 10.1038/ncomms5217
Article text (excluding photos or graphics) © Imperial College London. Photos and graphics subject to third party copyright used with permission or © Imperial College London. Communications and Public AffairsReporter
Press Office
