Artificial cell with multiple compartments could improve drug delivery
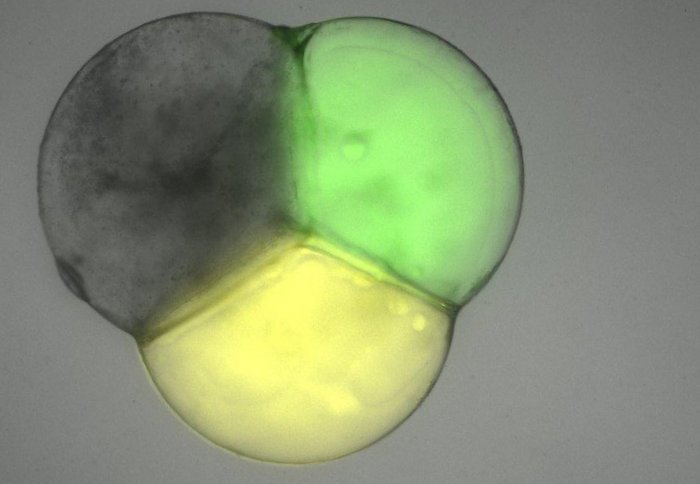
Microscope image of artificial cell compartments
Imperial scientists have made a new artificial cell that can compartmentalise its contents and set off a sequence of key biological processes.
The new artificial cell replicates how nature splits the contents of living cells for the first time by storing cell contents in separate compartments.
All cells have compartments, which each hold different contents and carry out diverse functions. For example, the mitochondria and the nucleus are compartments that perform distinct and well defined functions within one cell. The formation of these distinct compartments is called compartmentalisation and is a vital process in cell development and survival.
Imagine the cell is like a house. Every house has walls, rooms and doors. In the past we have only been able to build the outside of the house. But with our new technique, not only have we built the rooms inside the house, we’ve built the doors so you can travel through the house and get into the next room
– Dr Oscar Ces
Department of Chemistry
The new development by researchers from Imperial College London has the potential to unlock a wide variety of applications in the emerging field of ‘bottom-up’ synthetic biology, which rely on storing cell contents in different places. They describe their work today in a study in the journal Nature Communications.
The scientists were able to instruct each compartment individually and then merge them together to make a spatially complex artificial cell, which could carry out chemical processes that relied on transferring material from one compartment to the next.
The scientists are now working on applying their technique to manufacture designer molecules that use natural proteins and enzymes to create smart drug delivery systems.
Senior researcher Dr Oscar Ces from the Department of Chemistry and Institute of Chemical Biology at Imperial College London said: “Imagine the cell is like a house. Every house has walls, rooms and doors. In the past we have only been able to build the outside of the house. But with our new technique, not only have we built the rooms inside the house, we’ve built the doors so you can travel through the house and get into the next room.”
Dr Rob Law also from the Department of Chemistry and Institute of Chemical Biology at Imperial College London said: “So far we have been able to make artificial cells reproduce, make artificial cells communicate with each other, and even make artificial cells metabolise and produce their own energy. But these are ‘one-pot’ processes and bringing together isolated processes and functions within one cell has eluded us. So replicating natural cell compartmentalisation in an artificial environment is a big step forward for synthetic biology.”
Describing how the scientists manufactured these ‘multi-compartment vesicles’, lead author Yuval Elani, who is a PhD student in the Institute of Chemical Biology Centre for Doctoral Training, said: “We generated these unique structures using micro-droplets, which are made of water and oil. Membranes were constructed around each droplet, and by bringing several droplets together we ended up with units that contained several compartments.
“The fact that we could finely control the content and function of each compartment is key, as this allows them to be used as complex functional micro-machines in the future.”
Each compartment contained different chemicals and enzymes that carry out distinct biochemical processes. Once the compartments were made, the scientists used this separation of content to create and control a chain reaction of chemical processes that flowed between each compartment.
The first compartment contained lactase, which is an enzyme found in the human digestive system that breaks down sugar into simpler building blocks to give the cell energy. Once formed, the reaction product, in this instance glucose, travelled into the next compartment via a protein pore called alpha-hemolysin to set off the next chemical process.
In the second compartment glucose oxidase was present, which allowed the previously formed glucose to react with oxygen, to give hydrogen peroxide, which is an important chemical used for immune response.
And in the third compartment the enzyme horseradish peroxidase (HRP) was used to process the previously created hydrogen peroxide in order to generate the final product, an orange fluorescent molecule called resorufin.
The scientists used fluorescence microscopy to identify whether resorufin was produced. Their results showed that orange fluorescence was found at the end of the sequence proving that compartmentalisation had taken place and individual compartments had separate isolated processes, each relying on the product produced by enzymes in the previous compartment.
The research was funded by the Engineering and Physical Sciences Research Council (EPSRC) and the Biotechnology & Biological Sciences Research Council (BBSRC).
REFERENCE: Elani. Y et al. ‘Vesicle based artificial cells as chemical microreactors with spatially segregated reaction pathways’ Nature Communications. October 2014. DOI:10.1038/ncomms6305
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Press Office
Communications and Public Affairs
- Email: press.office@imperial.ac.uk
