Heart attack drug research awarded 2.7m pound funding
by Sam Wong
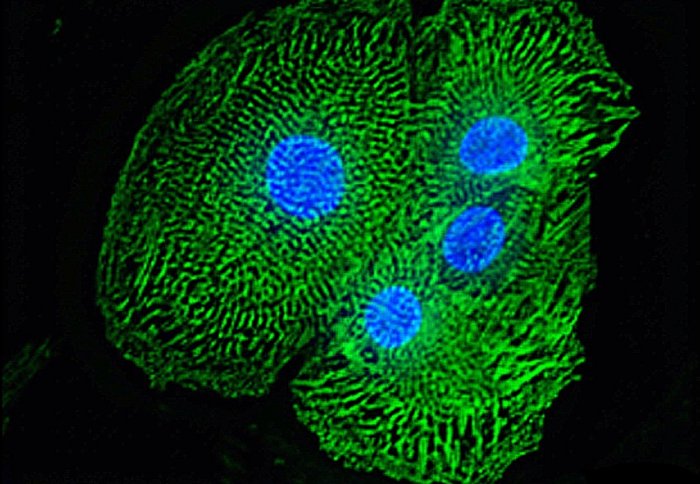
Heart muscle cells grown from human stem cells in the lab. Photo: Dr Evie Maifoshie
Imperial College London has been awarded a £2.7 million grant to develop drugs that lessen the damage caused by a heart attack.
The funding, from the Wellcome Trust’s Seeding Drug Discovery (SDD) initiative, will support two years of work on a programme of research that has already made important progress to identify a promising drug target in the heart and candidate molecules to control it.
A heart attack occurs when a clot blocks a blood vessel supplying the heart muscle. Starved of oxygen, the heart muscle cells produce stress signals that ultimately trigger cell death.
Treatments that restore blood flow, such as angioplasty, stents and clot-busting drugs, have been extremely successful at improving survival from heart attacks over the past 30 years. But survivors lose a significant part of their heart muscle irreversibly, which cannot be restored.
Professor Michael Schneider and his team at the National Heart and Lung Institute are aiming to develop drugs that could be administered in the early hours of a heart attack to prevent the death of heart muscle cells.
The group has identified an enzyme, MAP4K4, that appears to play a central role in triggering the death of heart muscle cells in response to stress signals. They have also developed potent and selective small molecules that inhibit this enzyme.
The new funding will allow the team to develop and improve these compounds further, test the molecules in human cells and in mice, and identify the most effective, safest candidates to take forward.
“There are no existing therapies that directly address the problem of muscle cell death,” said Professor Schneider. “This would be a revolution in the treatment of heart attacks.”
In developing countries, heart disease is becoming more common, but patients are less likely to have rapid access to heart attack treatment centres where they can be given an angioplasty to open the blocked vessel. “In those settings, drugs that lessen the damage to heart muscle could be all the more valuable,” he added.
Previous research in this area has not produced any drugs that have been successful in clinical trials, but Professor Schneider believes his project will be more fruitful. “Using the modern methods of molecular pharmacology, we’ve been able to ferret out a protein involved in making heart muscle cells sensitive to stress signals.”
Studying tissue samples from patients, the researchers found that MAP4K4 is activated in end-stage heart failure caused by heart attacks and other conditions. They also showed that it is activated in mice following a heart attack, and in heart cells subjected to stress signals in the lab.
Restoring blood flow in the heart – known as reperfusion – is necessary to limit the damage to heart muscle, but paradoxically, it also activates signalling proteins that cause further damage. The researchers showed in mice and in human tissue that if you raise the levels of MAP4K4, the cells are made more sensitive to stress signals. If you block MAP4K4, the cells are protected.
Some of the research has used heart muscle cells grown from human stem cells, a revolutionary platform for improving drug discovery. “One reason why many heart drugs have failed in clinical trials may be that they have not been tested in human cells before the clinic. Using both human cells and animals allows us to be more confident about the molecules we take forward,” said Professor Schneider.
The research in mice will use an advanced magnetic resonance imaging (MRI) scanner that enables the researchers to obtain precise measurements non-invasively, reducing the number of animals that need to be used and requiring less suffering.
The research to this point has been supported by the Imperial Confidence in Concept Scheme, set up with funds from the Medical Research Council, the National Institute for Health Research Imperial Biomedical Research Centre and Imperial Innovations. The scheme was designed to provide pilot funding to bridge the gap between discovery research and well-developed applications for drug development funding schemes like SDD. Professor Schneider is the British Heart Foundation Simon Marks Chair in Regenerative Cardiology and Director of Imperial’s British Heart Foundation Centre of Research Excellence.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Sam Wong
School of Professional Development