Ebola vaccine trials recruiting healthy volunteers
by Sam Wong
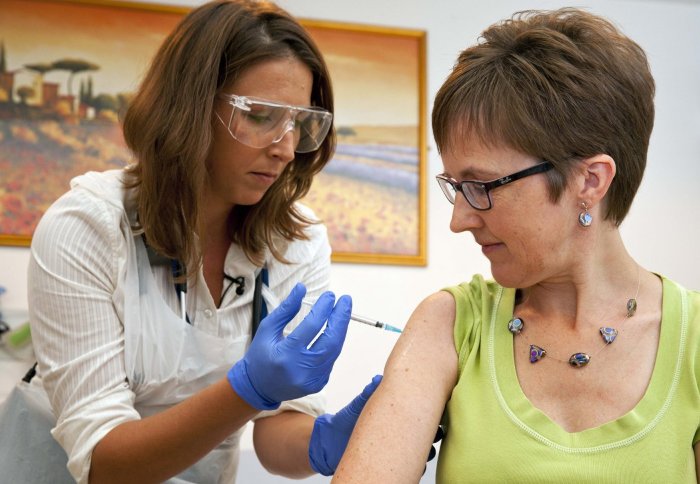
A volunteer receives an experimental Ebola vaccine in Oxford. Photo: Wellcome Images
Healthy volunteers are being sought to take part in clinical trials of three new Ebola vaccines in the UK.
Scientists at the University of Oxford and Imperial College London are conducting the trials as part of a global effort to develop and test vaccines for Ebola.
“This is a really unusual and exceptional situation where vaccines have moved into clinical trials very rapidly in response to the outbreak of Ebola in West Africa,” said Dr Saranya Sridhar, part of the study team, from the Department of Medicine at Imperial and the Jenner Institute at Oxford.
Although the Ebola outbreak appears to be stabilising, the researchers say it is important to continue developing and testing Ebola vaccines. “We still need to know a lot about what protects us against Ebola. At the same time, we need to develop a stockpile of vaccines that can be used in future outbreaks,” said Dr Sridhar.
Seven Ebola vaccines have begun clinical trials in the last year. A few are already in large, “phase III” trials in west Africa which are testing whether they protect people against Ebola following safety trials in Europe and the US.
The vaccines being tested at Oxford and Imperial are viral vector vaccines. This means they use weakened viruses to deliver a part of the Ebola virus into human cells to trigger a response by the immune system. This approach is relatively new, but has been trialled for other diseases such as malaria, tuberculosis, and influenza. As with other vaccines, the immune system keeps a memory of the foreign protein, enabling it to respond more effectively if it is encountered again.
The two trials, which are being led by Professor Adrian Hill of the Jenner Institute at Oxford University, are aiming to recruit 70 volunteers in total. Each trial will test a combination of two vaccines, one to prime the immune system and another given as a booster.
The researchers take blood samples to measure two types of immune response, antibodies and T cells, that might protect people against Ebola. They will also look for possible side effects.
The study visits in London will take place at the NIHR/Wellcome Trust Imperial Clinical Research Facility at the Hammersmith campus. Participants must be aged between 18 and 50, resident in the UK and registered with a GP. They will have to attend a number of visits at the trial sites in Oxford or London over a period of five to seven months. They must not have travelled to Ebola-affected countries in the last six months or have plans to travel to Ebola-affected countries.
Trial EBL04 is being funded by the Wellcome Trust and the Department for International Development. Trial EBL05 is being funded by GlaxoSmithKline and Johnson & Johnson.
To find out more about these or other trials recruiting healthy volunteers, call the Volunteer Co-ordinator on +44 (0)20 3313 8082, email Recruitment.ICRF@imperial.nhs.uk or visit the Imperial Clinical Research Facility website.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Sam Wong
School of Professional Development
