Star role for 'Wars2' gene in heart disease risk
by Kate Wighton
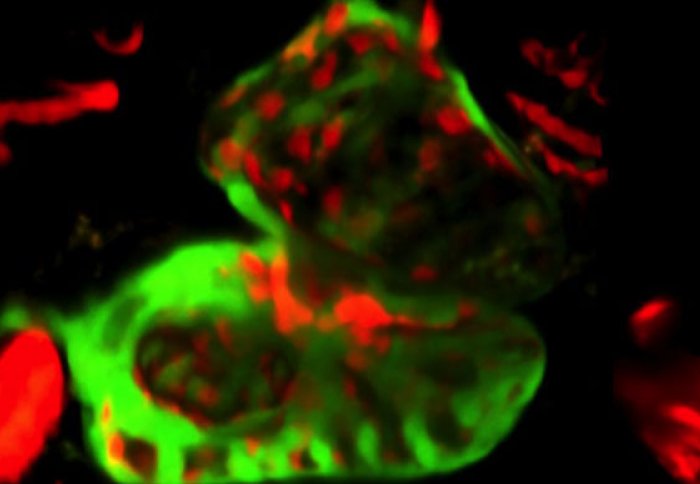
Zebra Fish heart with heart chamber cells in red and muscles cells in green
A gene called 'Wars2' has been pinpointed as a potential new target in treating heart disease, in research published today.
These latest findings, from Imperial College London researchers, are the first to show that Wars2 has a star role in driving the growth of blood vessels in the heart.
In some heart conditions, there are too few blood vessels to meet the heart’s energy demands. “If we can activate this gene, it may be possible to stimulate the growth of more vessels to treat heart disease. This would be a major step forwards,” says Professor Stuart Cook, from the National Heart and Lung Institute at Imperial, and who leads the Cardiovascular Magnetic Resonance Imaging and Genetics group at the MRC Clinical Sciences Centre.
Blood vessels form a transport system that supplies the body’s tissues and organs with critical nutrients and oxygen. New vessels are constantly produced to replace those that wear out, and to create new routes or diversions. This vessel growth, creating and maintaining the body’s internal road work system, is called angiogenesis.
If we can activate this gene, it may be possible to stimulate the growth of more vessels to treat heart disease. This would be a major step forwards
– Professor Stuart Cook
Study author
It’s not currently possible to treat disrupted angiogenesis in people with heart disease, because scientists do not fully understand how it functions. However, this study shows that Wars2 is critical. If its action is blocked, angiogenesis is impaired and this can cause disease. The suggestion is that Wars2 acts by integrating molecular signals that together stimulate angiogenesis. It may also direct the movement and growth of cells, particularly those in the sprouting tips and stalks of new vessels.
Angiogenesis is an intricate process that is difficult to study in beating heart tissue. The researchers therefore measured it indirectly, by monitoring blood flow through the heart. This provides an indication of the density of blood vessels, and how that is changing.
The research, which was published in the journal Nature Communications, was performed using heart tissue from rats, which are often used as a model to study heart conditions because the structure of their hearts is similar to ours. Early results also suggested that blood flow through a rat’s heart is determined by genetics, as it is in people. This makes the researchers confident that their findings will be relevant to patients.
The team identified particular rats that had reduced blood flow through the heart, an indicator of abnormally low angiogenesis, and analysed their genomes. This analysis showed that the genes which control angiogenesis are located in a particular region on the genome. They then narrowed this region down to the Wars2 gene itself.
To confirm the function of Wars2, they inhibited its action within the cells that line the vessels of the heart, in which angiogenesis takes place. This inhibition changed the cells’ shape and reduced their ability to proliferate. In this way, it directly impaired angiogenesis and reduced the number of small blood vessels, or capillaries, in the tissue.
Next the team explored the role of Wars2 in a living animal. They studied zebrafish, which have two-chambered hearts that are useful models of heart disease in people. It has previously been shown that mutations in genes similar to Wars2 can cause heart defects in zebrafish. In this study, the scientists inhibited Wars2 in the zebrafish embryos. This disrupted the normal interactions between the cells that line the chambers of the fish’s heart and those that make up the heart muscle (pictured). The researchers suggest that this leads to reduced vessel growth, which persists in adult fish.
They also inhibited Wars2 in adult fish, by adding molecules that block its action to the water in which they were kept. This reduced vessel growth throughout the fish’s upper body, or trunk, and caused fluid to build up around the fish’s heart. It was linked with early death.
Together, these results suggest that Wars2 is indeed needed for normal angiogenesis not only in the heart, but elsewhere in the body too. This builds on previous results, which have shown that a gene called VEGF (vascular endothelial growth factor) regulates angiogenesis in this way. The VEGF gene has been targeted by treatments for heart disease, which have reached clinical trials. It’s also targeted to treat certain cancers and eye diseases.
The findings may therefore have relevance beyond heart disease, according to the researchers. Wars2 has previously been associated with conditions that affect a variety of body tissues. For example, the inheritance of Wars2 has been linked to breast cancer, and the area in the genome where Wars2 sits has been associated with obesity and high blood sugar in people. Different versions of the gene could possibly be markers to predict the likelihood of a person developing a condition before symptoms develop.
Cancers and diabetic eye diseases can also hijack angiogenesis and up-regulate it to produce extra vessels, which allow them to suck up additional nutrients to fuel their spread. “If scientists could develop a drug to inhibit the gene, this may prevent this blood vessel formation in disease,” says Cook.
Adapted from a press release by Deborah Oakley, MRC Clinical Sciences Centre
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Kate Wighton
Communications Division