A new way to create synthetic proteins could lead to more flexible designs
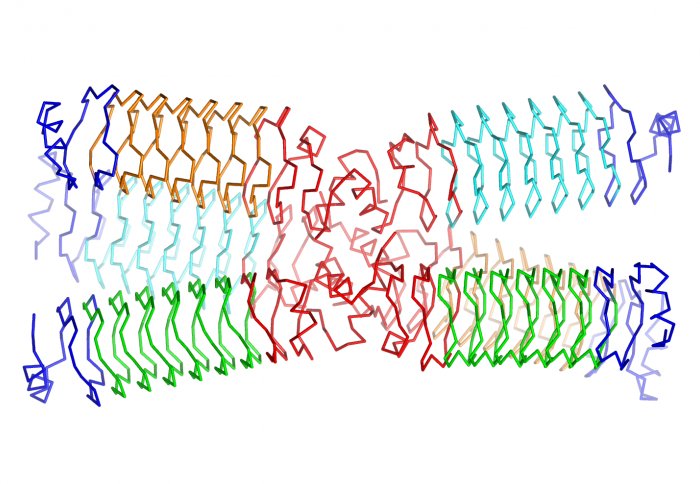
Structure of a designed protein
Building up proteins from scratch, rather than piecing together fragments of existing proteins, could make designing new nanomaterials easier.
Proteins perform a myriad of functions essential for life. They also make up important and useful biological materials, for example spider silk, which is exceptionally strong but still flexible.
The ability to design completely new proteins would help scientists create nanomaterials that, like spider silk, have a specific microstructure that confers useful properties.
Being able to construct proteins at the atomic level has a lot of potential and exciting applications, including synthetic enzymes and new nanomaterials.
– Professor Paul Freemont
Until now, new proteins have usually been designed by piecing together fragments of existing proteins in order to simplify the design process.
Now, a team led by researchers from Imperial College London has used a synthetic repeating protein scaffold as a base and shown that it is possible to add individual computationally designed modules, which can be chosen for their ability to perform a specific function. This gives biological engineers the possibility of designing new molecules from scratch.
The base scaffold is a new artificial repeating helix to which functional modules can be added. The team designed the structure on a computer, created it using synthetic genes, and then used a technique called X-ray crystallography to confirm they had built what they set out to.
Building complexity
Study leader Dr James Murray from the Department of Life Sciences at Imperial said: “Our system would allow designers to create proteins with atoms in specific places and build up complexity module by module, rather than designing the protein all at once.”
Dr James MacDonald from Imperial’s Centre for Synthetic Biology and Innovation added: “We have developed a new method for computationally designing brand new proteins that is potentially more flexible than taking sections from known proteins.”
The team’s first experimental results, published today in Proceedings of the National Academy of Sciences, added just one module to a helical scaffold as a proof that the system could work. Next, they want to add more loops to build up new functionality, and then test whether the synthetic proteins perform as expected.
Professor Paul Freemont, co-founder and co-director of the Centre for Synthetic Biology and Innovation at Imperial, said: “Being able to construct proteins at the atomic level has a lot of potential and exciting applications, including synthetic enzymes and new nanomaterials.
These could include improved nanowire batteries, where viruses are programmed to produce thin wires that increase the surface area and performance of batteries.”
-
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division