Six ways Imperial researchers are working to eliminate malaria

To mark World Malaria Day, we explore how Imperial scientists are tackling the disease as part of the College's new Network of Excellence in Malaria.
Malaria is caused by a parasite carried by certain species of mosquito, and killed an estimated half a million people last year. While the number of deaths from the disease is shrinking, there is still a long way to go before eradication, and there are significant challenges ahead.
At Imperial, we have a unique breadth of expertise from bench to bedside, parasite to vector. Working together, alongside our global partners, we believe the network can help realise the goal of an eventual world eradication of malaria.
– Dr Jake Baum
For example, the mosquitoes are becoming resistant to insecticide sprays, and the parasites themselves have evolved to evade our immune system, and are becoming resistant to antimalarial drugs used to treat people. No one solution alone can tackle all these challenges, and new innovations are required, along with the tools to identify which interventions are working.
Over 100 researchers at Imperial, from every faculty, work on different aspects of malaria. To bring this diverse group together, the Imperial College Network of Excellence in Malaria has been set up with the aim of combining scientific insights, technological innovations and evaluations of impact.
One of the network’s founders, Dr Jake Baum from the Department of Life Sciences, said: “The network provides an unsurpassed opportunity to harness the power of interdisciplinary research across the physical, natural, engineering and economic sciences together to tackle one of humanity’s greatest, and oldest, foes.
“At Imperial, we have a unique breadth of expertise from bench to bedside, parasite to vector. Working together, alongside our global partners, we believe the network can help realise the goal of an eventual world eradication of malaria."
We talked to six of the teams to find out more about the different plans of attack.
Genetic mosquito control
Professors Austin Burt and Andrea Crisanti from the Department of Life Sciences are focusing on the mosquitos that carry the disease. They aim to reduce local populations of the three species of mosquito (out of more than 3,500 species worldwide) that are responsible for most of the malaria transmission in Africa.
 To do this, they are genetically modifying the males of the species to bias the sex ratio of their offspring towards males (as males don’t bite and therefore don’t transmit the disease). They are also exploring the way to disrupt essential genes for female fertility, and to rapidly pass on this trait. In this way, populations will quickly diminish to low enough levels that they can no longer transmit the disease.
To do this, they are genetically modifying the males of the species to bias the sex ratio of their offspring towards males (as males don’t bite and therefore don’t transmit the disease). They are also exploring the way to disrupt essential genes for female fertility, and to rapidly pass on this trait. In this way, populations will quickly diminish to low enough levels that they can no longer transmit the disease.
The team have already had success in the lab at Imperial, and hope their innovation could one day be a key part of helping eradicate malaria. The team at Imperial are part of a large international project called Target Malaria, receiving core funding from the Bill and Melinda Gates Foundation.
They hope to start trials in three African countries in the future, but not before extensive and step-wise safety testing and engagement with local communities. As part of this process, Professor James Barlow from the Business School and Valentina Cisnetto have been investigating the economic, social and cultural challenges in introducing genetically modified mosquitoes into Burkina Faso.
Lab on a chip
Diagnosis is essential for malaria control, as is the need to identify drug-resistant strains of the parasite. Dr Pantelis Georgiou and Dr Jesus Rodriguez Manzano, from the Centre for Bio-inspired Technology at the Department of Electrical and Electronic Engineering, are exploring the use of miniature diagnostics. These so-called lab-on-a-chip technologies could provide fast, cheap testing for malaria infection and parasite drug resistance that could be deployed around the world.
Dr Rodriguez Manzano said: “In my opinion, a new generation of connected malaria rapid diagnostic tests for the detection and identification of parasite species, and rapid screening of emerging drug resistance strains, will play a critical role in the elimination of malaria and will become the backbone for clinical decision-making- as well as malaria control and prevention.”
Discovering why children are vulnerable
There are over two million new cases of malaria each year, and currently around half a million people die. The majority of people who die from malaria are children in sub-Saharan Africa. Dr Aubrey Cunnington, from the Department of Medicine, wants to understand why many children with malaria develop life-threatening complications, and to find better treatments to prevent their deaths.
Dr Cunnington and his team use donated blood samples from African children with malaria to identify the mechanisms that are most likely to be causing severe disease, and then test whether they can block these effects in a variety of model systems in the lab.
Dr Cunnington said the network is important for making sure eradication efforts are driven forward: “Eradicating malaria, getting rid of the disease completely from the world, is a massive and complex challenge because it requires political will, sustained funding, sustained implementation, and an arsenal of new weapons which can be used when the parasites or mosquitoes evade our existing strategies.”
Getting inside the mind of the parasite
Dr Jake Baum and his team, from the Department of Life Sciences, tackle the parasite head on. They aim to discover as much detail as possible about the fundamental wiring of the parasite – from how it grows and divides to how it targets and invades cells – in order to find new ways of stopping it dead in its tracks.
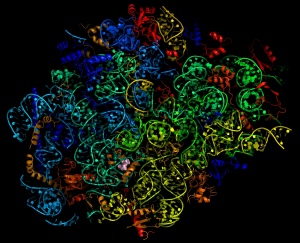
Map of the parasite ribosome
They have already built a molecular map of the parasite ribosome – the cell machinery that manufactures proteins – which is a potential future drug target. Following this, they have for the first time shown how one of the long-standing antimalarials could be redesigned to more effectively target the parasite in an attempt to overcome emerging resistance.
The team have also looked at how the parasite is able to break into red blood cells – the phase of the disease that causes all physical symptoms. They found that as well as pushing with its own molecular motor, the parasite changes the properties of the red blood cells themselves, making their membranes more flexible and easier to bypass.
Nanotech to the rescue
Taking a techy approach, Dr Adrian Najer focuses on engineering nanotechnological materials to build devices for malaria diagnostics, treatment and even protection from future infections. “Given the limited success with experimental vaccines, this is an exciting area for research towards an alternative option to protect people from future infections,” he said.
Dr Najer will also adapt analysis techniques currently used to characterise materials to provide a valuable research tool for basic biology questions about malaria. His work crosses faculties, working with researchers in the Department of Life Sciences as well as the Department of Materials. He said: “I believe that keeping up the efforts in all areas involved in malaria eradication is a must, because the failure of one area can lead to the collapse of the whole and a resurgence of malaria, which is not acceptable.”
Predicting outcomes
There are a lot of new interventions being developed, but it is difficult to know how effective they will be in the field. Dr Thomas Churcher, from the School of Public Health, uses mathematical models to predict how good they might be in different locations with different situations.
 Dr Churcher takes data from laboratory experiments and simulates their effectiveness in certain areas, predicting how many lives might be saved. He said: “We do mathematical modelling, but we need to coordinate closely with people doing lab work that directly feeds into our models. The more we can get that interaction, the more accurate our prediction will be.”
Dr Churcher takes data from laboratory experiments and simulates their effectiveness in certain areas, predicting how many lives might be saved. He said: “We do mathematical modelling, but we need to coordinate closely with people doing lab work that directly feeds into our models. The more we can get that interaction, the more accurate our prediction will be.”
He also works with laboratory experiments run at Imperial investigating aspects of the disease, such as recent work looking into how the number of malaria parasites in a mosquito bite changes the chance of being infected, which has implications for vaccines.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division