Regenerating the heart: A Q&A with Dr Susanne Sattler

Dr Susanne Sattler, National Heart and Lung Institute
Around 915,000 people in the UK today have had heart attacks, which deprive heart cells of oxygen, causing them to die and be replaced by scar tissue.
Scar tissue helps maintain the structure of damaged hearts, but when the scarring is severe it can weaken the heart muscle and lead to heart failure.
Dr Susanne Sattler from the National Heart and Lung Institute, Imperial College London, conducts research into what prevents the heart cells from re-growing, or regenerating, after trauma such as heart attack leading to heart failure.
Dr Sattler spoke with Caroline Brogan about her recently published work.
Can you explain some of the challenges involved in treating heart attacks?
To encourage optimum heart function after a heart attack, scientists in our department and around the world are working on a number of regenerative therapies to help cell growth in damaged hearts, for example with stem cells.
– Dr Susanne Sattler
National Heart and Lung Institute
The way we treat heart attacks has significantly improved in the last decade, and although survival rates are very high, one in five will subsequently develop fatal heart failure. There is a wide range of drugs available to treat heart failure, but no absolute cure.
Unlike other organs such as the liver, the heart is notoriously bad at regenerating after injury, and damaged tissue turns into stiff scars. To encourage optimum heart function after a heart attack, scientists in our department and around the world are working on a number of regenerative therapies to help cell growth in damaged hearts, for example with stem cells.
Any tissue damage in the heart will cause inflammation, which is an immune response that helps to close wounds and remove dead cells to allow new tissue to grow. However, after severe tissue trauma, as we see after a heart attack, inflammation may be excessive and can activate other immune cells which recognise heart proteins as harmful. This phenomenon, otherwise known as an autoimmune response, causes the patient’s own immune system to attack healthy heart tissue and cause more damage.
How does this hinder patients’ rehabilitation?
Once it begins, an autoimmune response targeting the heart is unlikely to subside by itself and will cause more and more damage, even in previously healthy areas of the heart. Antibodies, which are molecules which under healthy conditions recognise and attack ‘foreign’ cells, mistakenly ‘mark’ heart cells as harmful so that the immune system ‘knows’ to destroy them. It is easy to see how this is detrimental to the recovery of healthy heart function, and can, in turn, undo the positive effects of regenerative therapies.
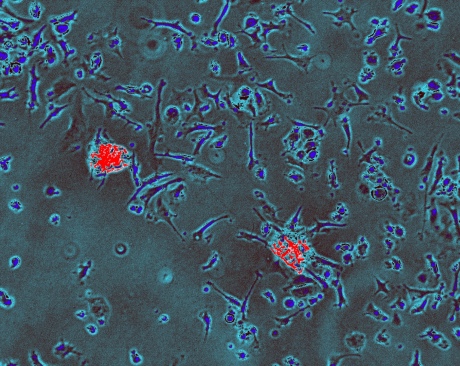
Immune cells (blue) engulfing two unhealthy heart cells (red) and breaking them down for ‘disposal’. Credit: Stephen Rothery, Facility for Imaging by Light Microscopy, Imperial College London
What is your current research in the area of tissue regeneration?
I am part of the UK Regenerative Medicine Platform Immunomodulation Hub, which aims to address immunological questions in regenerative medicine. I also work closely with the British Heart Foundation’s Regenerative Medicine Centre, led by Professor Sian Harding from Imperial’s National Heart and Lung Institute, which focuses on cardiac regeneration.
The immune system is mind-bogglingly complex, and we’ve come a long way, but a lot more basic, or fundamental, research is needed to achieve our goal.
– Dr Suanne Sattler
National Heart and Lung Institute
My research focuses on the crosstalk between the heart and the immune system, the normal and healthy immune response to damage, and the effect of autoimmunity on the heart. One specific project in the group currently investigates the role of a subset of immune cells, called dendritic cells, in the heart and how we can use them to stop autoimmune responses and induce tolerance, which after a heart attack effectively means ‘blinding’ the immune system against the heart again. If we are able to do this, this will have widespread implications in all sorts of autoimmune diseases.
My colleagues and I recently published a perspective article to raise awareness about the dangers of ongoing immune-mediated damage after heart attack, and its potential to cause regenerative therapy to fail. We argue that this immune response might be a prominent reason for the disappointing performance of regenerative therapies so far.
How can we improve knowledge of tissue regeneration and prevent further preventable tissue damage?
The immune system is widely considered a machinery to defend us from infectious diseases, which is certainly the case. However, the immune system is also crucial for developing, maintaining, and repairing tissues – hence why we need it on our side when trying to grow new heart tissue
The immune system is better known for protecting us from infectious diseases and cancer, as seen in this image of immune cells breaking down a cancer cell.
We now need to work out which immune cell to target at what exact window in time. The immune system is mind-bogglingly complex, and we’ve come a long way, but a lot more basic, or fundamental, research is needed to achieve our goal. The current research funding climate understandably favours studies which have direct patient benefit in the nearest possible future, but in my opinion, this needs to be balanced with studies aimed at understanding the underlying biological processes.
What’s next for the field of regenerative medicine?
Most regenerative therapies are based on similar principles: replace the damaged tissue, and prevent the immune system rejecting the transplanted cells or materials.
However, these therapies rely on the assumption that the damaging event, i.e. the heart attack itself, is over, and don’t consider that the heart might be suffering continued damage from autoimmunity. Regenerative therapies will inevitably fail if the tissue is still under attack.
My colleagues and I therefore believe the next crucial step to ensure the success of regenerative therapies is to design combination therapies that repair existing damage, as well as prevent further damage by restoring immune system tolerance for the cells in question. This is as true for any tissue and organ as it is for the heart.
“The adaptive immune response to cardiac injury—the true roadblock to effective regenerative therapies?” by Susanne Sattler, Paul Fairchild, Fiona M. Watt, Nadia Rosenthal and Sian E. Harding. Published in NPJ Regenerative Medicine on 19 June 2017
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Caroline Brogan
Communications Division