Jan 2019 - Article in Chemical Science Published

Our paper describing an investigation of the acid catalysed cyclocondensation reaction to form furanochromanes by Chris Nielsen is published.
Chris D-T. Nielsen, Wouter J. Mooij, David Sale, Henry S. Rzepa, Jordi Bures , Alan C. Spivey 'Reversibility and reactivity in an acid catalyzed cyclocondensation to give furanochromanes - a reaction at the "oxonium-Prins' vs. "ortho-quinone methide cycloaddition' mechanistic nexus', Chem. Sci. 2019, 10, 406-412 DOI: 10.1039/C8SC04302G
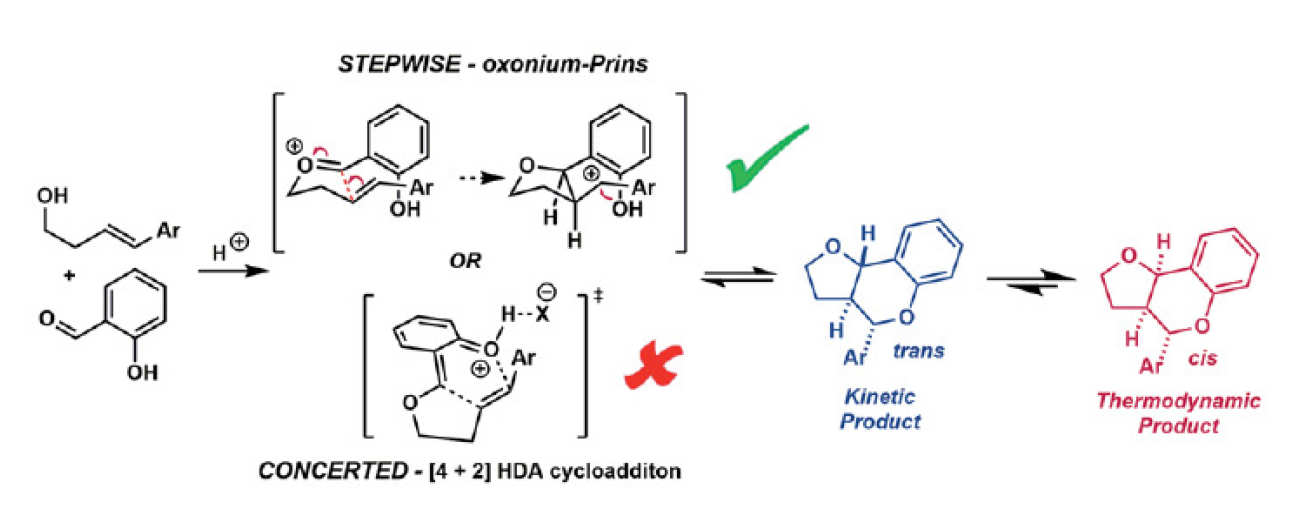
In this paper we report a combined experimental and computational investigation of the acid catalysed cyclocondensation reaction between styrenyl homoallylic alchohols and salicycaldehdyes to form furanochromanes. We disclose a previously unreported isomerisation of the ‘unnatural’ trans-fused products to the diastereomeric ‘natural’ cis-fused congeners. Notwithstanding the appeal of assuming this corresponds to endo to exo isomerisation of Diels-Alder (D-A) adducts via concerted retro-cycloaddition/cycloaddition reactions of an in situ generated ortho-quinone methide with the styrenyl alkene, our combined Hammett/DFT study reveals a stepwise Prins-like process via discrete benzylic carbocation intermediates for all but the most electron deficient styrenes. As these reactions fortuitously lie at the intersection of these two mechanistic manifolds, it allows us to propose an experimentally determined indicative rho+ value of ca. -3 as marking this nexus between a stepwise Prins-type pathway and a concerted cycloaddition reaction. This value should prove useful for categorising other reactions formally involving ‘ortho-quinomethides’, without the need for the extensive computation performed here. Logical optimisation of the reaction based upon the mechanistic insight led to the use of HFIP as an additive which enables exclusive formation of ‘natural’ cis-fused products with a .100-fold reaction rate increase and improved scope
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Professor Alan C Spivey
Department of Chemistry