Jan 2019 - Article in Angew. Chem. Published

Alex Boddy's paper on the synthesis of various nitrogen heterocycles by diazo-heterocycle 'stitching' is published.
Alexander J. Boddy, Dominic P. Affron, Chris J. Cordier, Emma L. Rivers, Alan C. Spivey and James Bull 'Rapid Assembly of Saturated Nitrogen Heterocycles in One-Pot: Diazo-Heterocycle "Stitching" by N-H Insertion and Cyclization', Angew Chem Int Ed Engl, 2019, 58, 1458-1462 DOI: 10.1002/anie.201812925
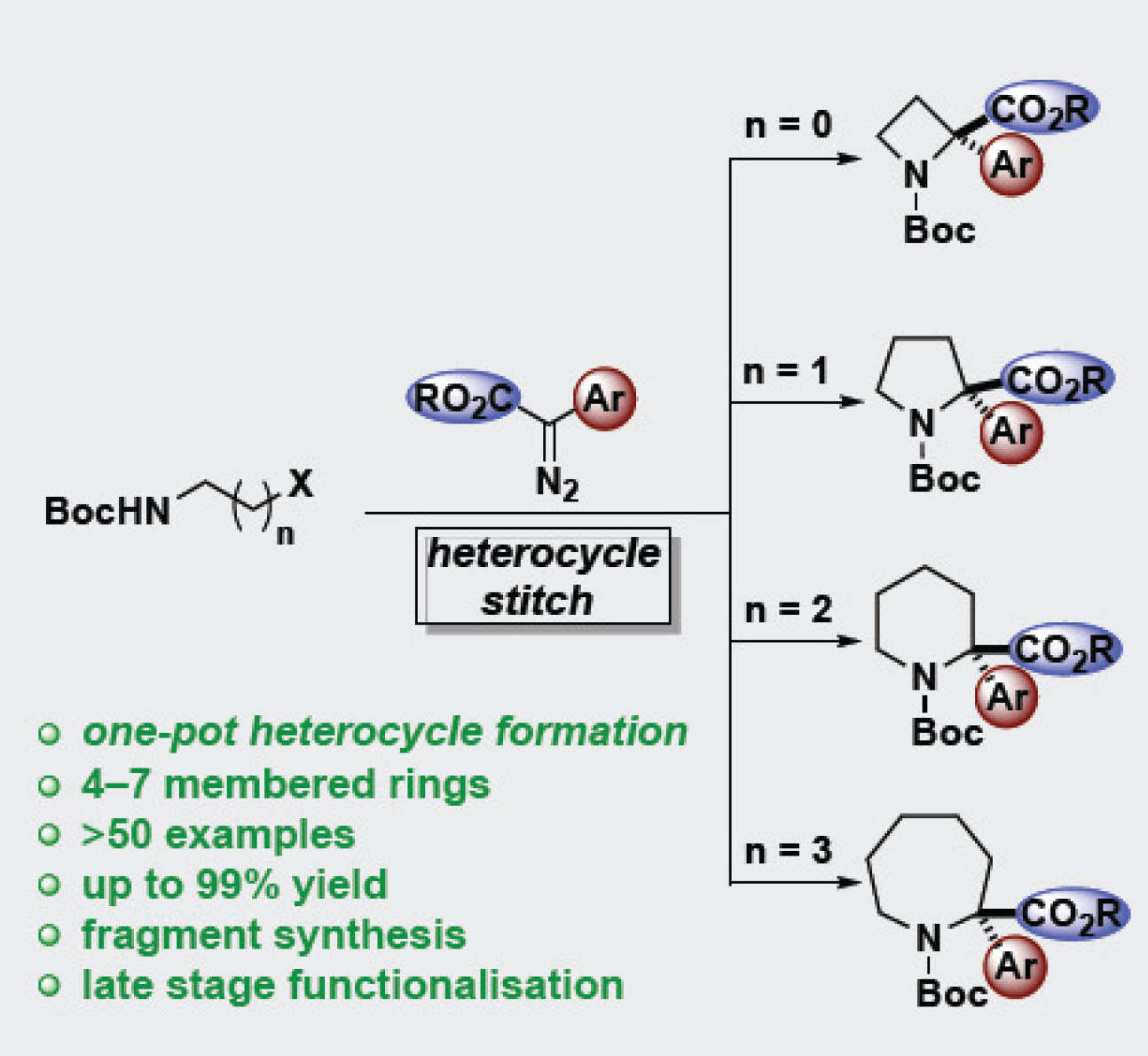
Alex is one of Dr James Bull's students, jointly also supervised by Alan Spivey and Chris Cordier. Here, he describes a strategy for the preparation of 2,2-disubstituted azetidines, pyrrolidines and piperidines, bearing ester and diverse aryl substituents. A one-pot rhodium catalyzed N–H insertion and cyclization sequence couples diazo compounds with linear alpha,omega-aminohalides to rapidly assemble the 4-, 5- and 6-membered saturated nitrogen heterocycles in excellent yields. Fifty (50) example have been prepared, including with functionalized diazo compounds and those derived from biologically active compounds. The products can be functionalized to afford alpha,alpha-disubstituted amino acids and applied to fragment synthesis.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Professor Alan C Spivey
Department of Chemistry
Dr Christopher J. Cordier
Department of Chemistry
Dr James A Bull
Department of Chemistry