Mar 2019 - Article in Org. Lett. Published

Alex Boddy's paper on the ring expansion of 2-ester-2-aryl-azetidine carbamates to 6,6 Disubstituted 1,3-Oxazinan-2-ones has been published.
Alexander J. Boddy, Christopher J. Cordier, Kristin Goldberg, Andrew Madin, Alan C. Spivey and James A. Bull 'Acid-Mediated Ring-Expansion of 2,2-Disubstituted Azetidine Carbamates to 6,6-Disubstituted 1,3-Oxazinan-2-ones' Org. Lett. 2019, 21, 1818-1822. DOI: 10.1021/acs.orglett.9b00407
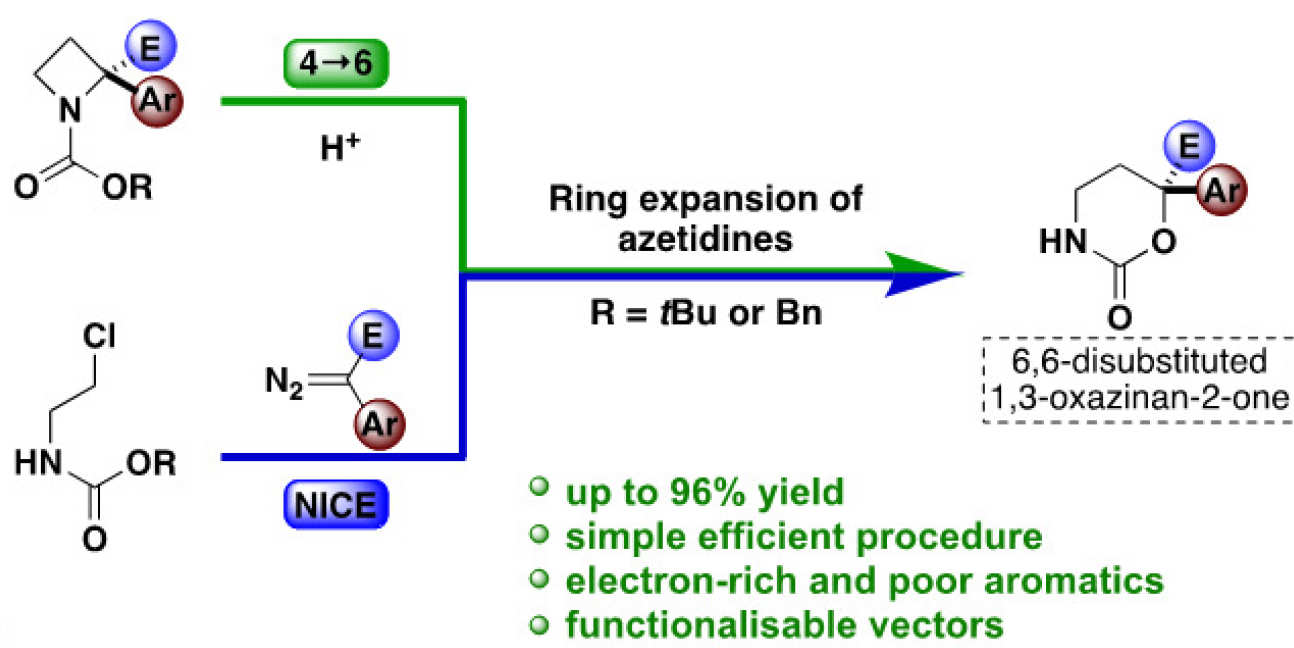
Alex is one of Dr James Bull's students, jointly also supervised by Alan Spivey and Chris Cordier. Here, he relates how the ring expansion of 2-ester-2-aryl-azetidine carbamates can be achieved using Brønsted acids to form 6,6-disubstituted 1,3-oxazinan-2-ones. The reaction is rapid at room temperature with Boc or Cbz derivatives, and proceeds with excellent yield (up to 96%) and broad substrate scope. Derivatives of drug compounds and natural products are incorporated. The combination of this ring expansion in a 3-step N–H insertion/cyclization/expansion (NICE) sequence is applied to directly access medicinally relevant scaffolds from acyclic precursors.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Christopher J. Cordier
Department of Chemistry
Dr James A Bull
Department of Chemistry
Professor Alan C Spivey
Department of Chemistry