The good, the bad and the ugly: chemical probes for human NMT
A new publication from the Tate Group both validates and invalidates chemical probes for human N-myristoyltransferase.
Well-characterised, potent and selective chemical probes for specific enzyme targets are essential tools in cell and chemical biology, and contribute greatly to the identification and validation of potential drug targets in human disease. However, compounds incorrectly claimed as selective due to a lack of proper validation produce incorrect results through off-target mechanisms, and can be deeply misleading in cell biology. Validation and invalidation of probes is thus essential to identify which studies are correct, and which are compromised by the use of an invalid probe.
In a new paper in Cell Chemical Biology Wouter Kallemeijn and Gregor Lueg, together with Monica Faronato, Kate Hadavizadeh, Andrea Goya Grocin and researchers at the Francis Crick Institute, have undertaken the first head-to-head comparison of chemical probes for N-mristoyltransferase (NMT). NMT is an enzyme that catalyses a post-translational lipid modification of proteins that is universally conserved in all eukaryotes, and is a well-validated drug target in trypanosomiasis, malaria and viral infections, as well as an emerging target in cancer.
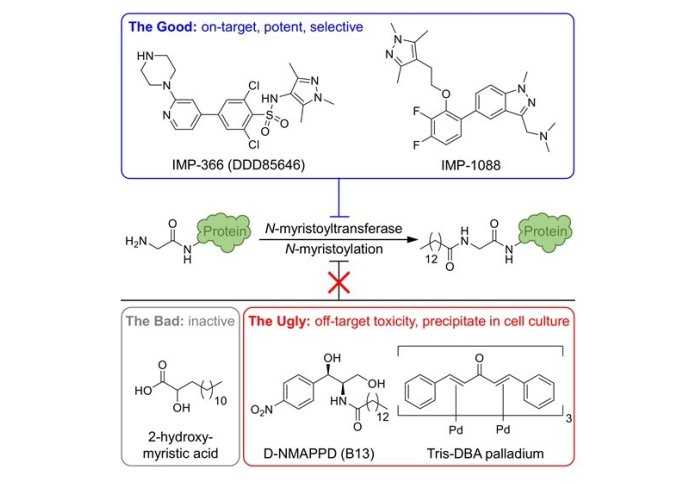
Wouter and Gregor identified two classes of selective probe for human NMT, including our previously published compound IMP-1088, and demonstrated that these compounds act on target through a comprehensive study in multiple cell types, using seven orthogonal approaches to validate these probes (enzyme assays, cell cycle analysis, apoptosis assays, chemical tagging, biomarker assays, whole proteome proteomics and protein synthesis dynamics). Importantly, their work also identified three off-target compounds which are widely but incorrectly used as probes for human NMT, all of which showed either inactivity or off-target toxicity, and no evidence for engagement of NMT as a target in any cell line tested. These compounds include 2-hydroxymyristate, D-NMAPPD and Tris-DBA palladium.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Jennie Hutton
Department of Chemistry