Screening for rare but important disease ‘biomarkers’ gets an accuracy boost
Researchers have created a system that can detect and quantify small and rare biological molecules that are important for detecting disease early.
Certain molecules in biological fluids like blood and urine can be indicators of disease, especially if they become more prevalent. However, in the early stages of disease these ‘biomarkers’ are rare and can be difficult to detect.
It is a simple way to detect molecules much smaller than the size of the nanopore without loss of signal to noise. Professor Joshua Edel
However, they are difficult to separate from the background of so many other biological molecules in fluids. Now, researchers from the Department of Chemistry at Imperial have invented a system that can identify and measure individual biomarkers in human serum (fluid from blood) and urine.
Detecting important biomarkers in lower concentrations will allow patients to be treated earlier for diseases including some cancers and neurological disorders, which could increase the survival rate.
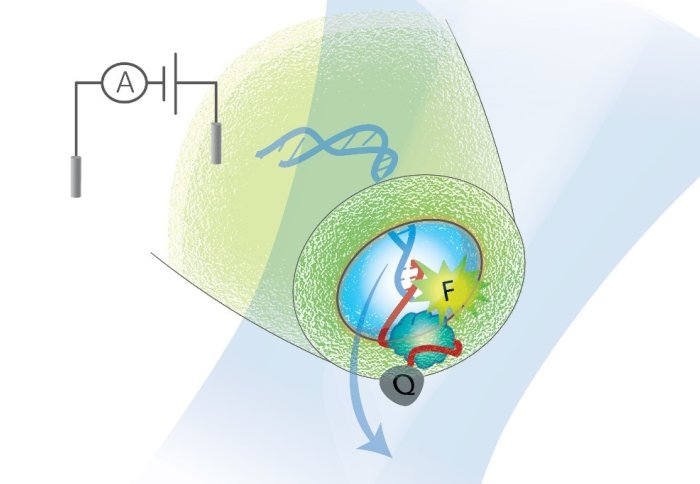
Their system, published this month in Nature Communications, tags biomarkers of interest and detects them with a nanopore – a hole a few billionths of a meter across that measures the change in electrical current as a molecule passes through.
Overcoming false positives
Previously, the team have used nanopores to detect biomarkers such as small DNA strands and single proteins. However, they have faced problems when the biomarkers are much smaller than the nanopore, meaning they may still be missed by the system.
This is particularly true when there are a lot of other small molecules that are not useful to detect, meaning the ‘noise’ outweighs the ‘signal’.
To overcome this, they created DNA carriers – special strands of DNA with sections designed to detect biomarkers of interest. Once these carriers find a biomarker they bind with it, allowing it to be more easily detected by the nanopore.
However, the DNA carriers would sometimes spontaneously ‘knot’, twisting themselves so it appeared they were attached to a biomarker. This could create ‘false positives’ – detections of biomarkers that weren’t really there.
Finding the signal in the noise
Now, they have improved the DNA carrier system by pairing it with fluorescence detection. When the DNA carrier binds with a biomarker it releases a burst of light, which tells the system that a biomarker is attached. It then passes through the nanopore, where the biomarker is read using its electrical signal.
Co-author Professor Joshua Edel said: “We demonstrated a system that can discriminate traditionally difficult biomarkers such as single proteins directly in biological fluid by quantifying simultaneous electrical and optical detection. It is a simple way to detect molecules much smaller than the size of the nanopore without loss of signal to noise.”
“The whole project was only made possible by the unique abilities and enthusiasm of the young team members, including Shenglin Cai and Dr Jasmine Sze, who all have diverse expertise and backgrounds.”
-
‘Small molecule electro-optical binding assay using nanopores’ by Shenglin Cai, Jasmine Y. Y. Sze, Aleksandar P. Ivanov & Joshua B. Edel is published in Nature Communications.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division