New film of immune system killing bacteria could point to new therapies
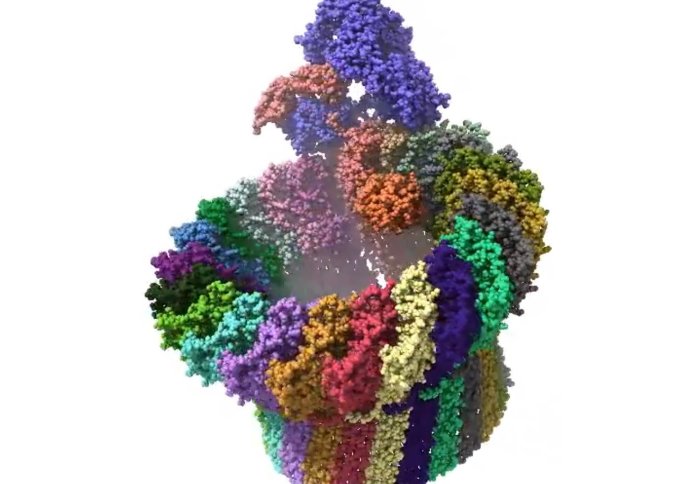
High-resolution image of a membrane attack complex
New film showing how our immune system attacks bacteria may guide the development of new therapies that harness the immune system against infections.
To kill bacteria in the blood, our immune system opens deadly ‘bullet holes’ in their membranes, causing them to burst and die.
The holes are created by structures called membrane attack complexes (MACs). Researchers have been trying to find out exactly how MACs work in order to potentially harness the process and supercharge the immune system.
Now, a research team led by scientists at UCL and including Imperial College London researchers have filmed MACs opening holes in bacteria membranes for the first time.
Film of MACs opening holes. Credit: Edward S. Parsons et al.
Their research, published in Nature Communications, provides us with a better understanding of how the immune system kills bacteria but also reveals why our own cells are able to avoid being punctured by MACs.
How bullet holes spread
The results may guide the development of new therapies that harness the immune system against bacterial infections, and lead to strategies that repurpose the immune system to act against other rogue cells in the body.
In earlier research, the scientists imaged the hallmarks of attack in live bacteria, showing that MACs result in ‘bullet holes’ that spread across the cell membranes of bacteria. The holes are incredibly small with a diameter of just 10 nanometres – about 1/10,000 of the width of a human hair.
Imperial researcher Dr Doryen Bubeck and her team from the Department of Life Sciences previously imaged MACs in high resolution using a technique called cryo electron microscopy. These snapshots provide valuable detail about the structure of the MAC, but not how they change over short time scales.
For the new study, the researchers mimicked how the MACs form these deadly holes, using a model bacterial surface, and took lower-resolution images at regular intervals. By combining structural insights from the high-resolution images and timely information from rapid movies, they were able to track each step of the process.
A reprise for the body's own cells
The team found that shortly after each hole started to form, the process stalled, offering a reprise for the body’s own cells. The team think the pause gives our own body’s cells time to signal to the MAC that they are not invaders, by showing them a specific protein.
We have begun to answer the question of how our immune system attacks invading bacteria without harming our own cells. Dr Doryen Bubeck
Dr Edward Parsons, from the London Centre for Nanotechnology at UCL, said: “It appears as if these nanomachines wait a moment, allowing their potential victim to intervene in case it is one of the body’s own cells instead of an invading bug, before they deal the killer blow.”
The MAC process pauses as 18 copies of the same protein are needed to complete a hole. Initially, there’s only one copy that inserts into the bacterial surface, after which the other copies of the protein slot into place much more rapidly.
Combining imaging
To film the immune system in action at nanometre resolution and at a few seconds per frame, the scientists used atomic force microscopy. This type of microscopy uses an ultrafine needle to feel rather than see molecules on a surface, similar to a blind person reading Braille.
The needle repeatedly scans the surface to produce an image that refreshes fast enough to track how immune proteins get together and cut into the bacterial surface.
These images were then combined with insights from the high-resolution images of the MAC produced by Dr Bubeck’s team to determine how the structure changes as the holes is built.
Dr Bubeck said: “By combining the structural information from our incredibly detailed snapshots with the series of images that stitch together to show a movie of what happens over time, we have begun to answer the question of how our immune system attacks invading bacteria without harming our own cells.”
The research also involved scientists from the Swiss Federal Institute of Technology in Lausanne and the University of Leeds and was funded by the Biotechnology and Biological Sciences Research Council, Medical Research Council, Engineering and Physical Sciences Research Council and the European Union.
-
‘Single-molecule kinetics of pore assembly by the membrane attack complex’ by Edward S. Parsons, George J. Stanley, Alice L.B. Pyne, Adrian W. Hodel, Adrian P. Nievergelt, Anaïs Menny, Alexander R. Yon, Ashlea Rowley, Ralf P. Richter, Georg E. Fantner, Doryen Bubeck, and Bart W. Hoogenboom is published in Nature Communications.
Based on a press release by UCL.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division