New Publication in Organic Letters
by Anna Barnard
Our new publication in Organic Letters is now out!
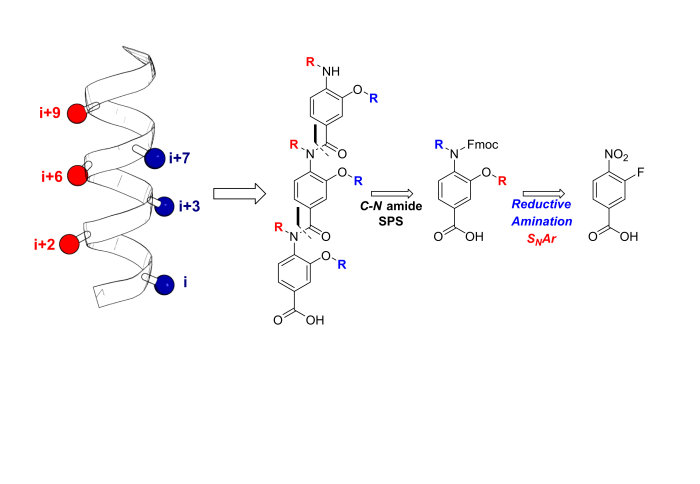
Our new paper entitled 'Design, Synthesis and Conformational Analysis of Oligobenzanilides as Multi-Facial alpha-Helix Mimetics' is now out in Organic Letters:
https://pubs.acs.org/doi/10.1021/acs.orglett.9b01115
In this paper the design, synthesis, and conformational analysis of an oligobenzanilide helix mimetic scaffold capable of simultaneous mimicry of two faces of an α-helix is reported. The synthetic methodology provides access to diverse monomer building blocks amenable to solid-phase assembly in just four synthetic steps. The conformational flexibility of model dimers was investigated using a combination of solid and solution state methodologies supplemented with DFT calculations. The lack of noncovalent constraints allows for significant conformational plasticity in the scaffold, thus permitting it to successfully mimic residues i, i+2, i+4, i+6, i+7, and i+9 of a canonical α-helix.
The synthetic work and conformational analysis was performed by Dr Theo Flack during his PhD and DFT calculations were performed in collaboration with Dr Charles Romain. Crysallography and NMR assistance were provided by Andrew White and Pete Haycock. Congratulations to all involved!
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Anna Barnard
Department of Chemistry