It takes three to make oxygen from water on metal oxides under sunlight

A combined experimental and computational study on the water oxidation mechanism found water splitting on hematite changes with light intensity.
Solar water splitting provides a way to convert intermittent solar energy into chemical fuels. Plants use an enzyme called photosystem II (PSII) to absorb light and split water, extracting electrons and protons from water molecules and generate atmospheric oxygen that sustains life on Earth. The electrons are ultimately used to make adenosine triphosphate (ATP), a chemical fuel that is stored and used when needed by the plant.
Sustainable approaches to generate fuels aim to mimic photosynthetic mechanisms to generate fuels from sunlight and water. An outstanding challenge is to understand the functionality of earth-abundant materials that can absorb sunlight and extract electrons from water to generate fuels; either by combining protons and electrons for hydrogen generation, or using the electrons and protons to convert carbon dioxide into hydrocarbon fuels.
Hematite (reddish iron oxide, otherwise known as rust) is a promising material for solar water splitting (i.e. the conversion of water into hydrogen fuel using sunlight), although its performance is below its theoretical maximum and its catalytic functionality remains to be understood at the molecular level.
Underlying mechanisms
Extensive studies have been carried out to identify the possible reaction intermediates formed during the water splitting at the oxide/water interface. However, a fundamental understanding of the reaction mechanism is lacking, preventing the rational development of more efficient water splitting devices.
A recent study by James Durrant from Imperial College London with Victor Batista (Yale), Michael Grätzel (EPFL) and Erwin Reisner (University of Cambridge) and Andreas Kafizas (Imperial) sheds light on this water splitting mechanism of water oxidation occurring at the metal oxide/water interface.
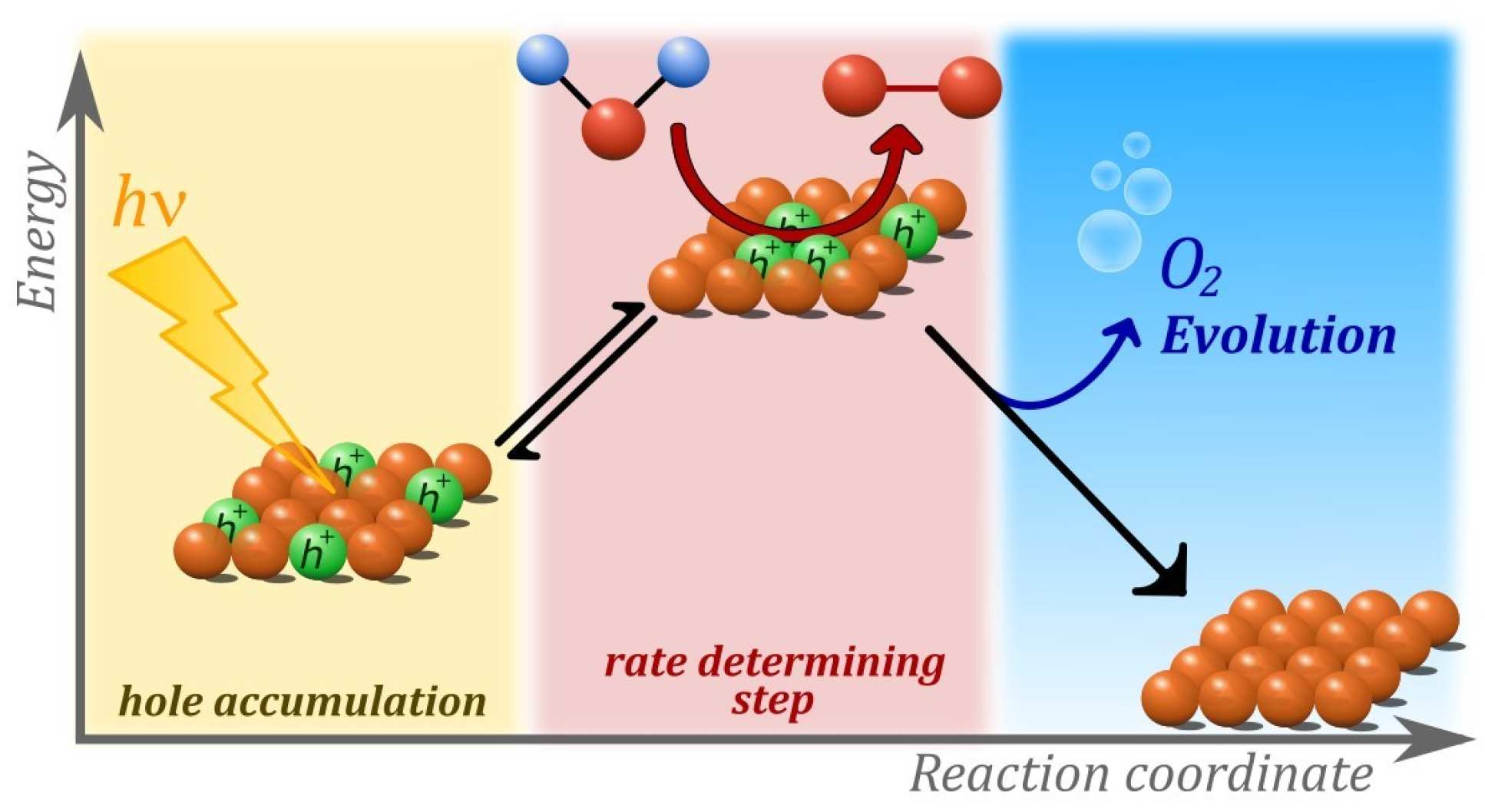
A combined experimental and computational study on the water oxidation mechanism found the mechanism of water splitting on hematite changes with light intensity. Strikingly, the rate of water oxidation increases with the third power of the surface hole density, providing valuable insight into the nature of the processes driving the reaction mechanism.
The experimental studies, undertaken by Camilo Mesa and Laia Francàs in the Durrant group, focused on understanding the unprecedented third-order kinetics of water oxidation under 1 sun irradiation. Experimental studies, combining electrochemical and spectroscopic measurements, shed light into the kinetics of three redox equilibria between photogenerated holes and the water oxidation catalytic centre.
These equilibria, now understood as an accumulation process, was found to reduce the activation energy of the water oxidation and appear to be central to understanding the function of water splitting devices based on hematite, as well as other metal oxide photoanodes.
Combined computational and experimental studies
James Durrant, whose group led the experimental work in this study, says: "Making oxygen from water requires multiple oxidations. Experimentally the key to our study has been using optical absorption spectroscopy to measure how the kinetics of water oxidation change as we accumulate more holes on the hematite surface.
"This has allowed us to determine rate laws and rate constants for the reaction – for example determining how many holes have to come together to access the rate-limiting step of the reaction, and determining the activation energy for the reaction."
Computational work performed by Dr Ke Yang in the Batista group identified isolated catalytic sites under low light intensity and an Fe(OH)-O-Fe(OH) catalytic core that is able to build enough oxidation power as to extract electrons from water by accumulating up to three oxidizing equivalents (missing electrons or ‘holes’) under 1 sun operating conditions.
Such a mechanism parallels the proposed activation of the catalytic site in PSII, despite the remarkable differences between the composition and structure of hematite and the biological enzyme.
Professor Victor Batista, who led the computational work in this study, says: "The integration of computational and experimental work has been essential for elucidating the nature of the catalytic sites on rather complicated metal oxide surfaces and the dependency of the reaction mechanism under low and high light intensity conditions.”
Corresponding authors for the study are Professor James Durrant at Imperial College London and Professor Victor Batista at Yale. The Imperial researchers are from the Department of Chemistry and the Centre for Plastic Electronics.
-
'Multihole water oxidation catalysis on haematite photoanodes revealed by operando spectroelectrochemistry and DFT' is published in Nature Chemistry.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Mr Camilo A. Mesa
Department of Chemistry