Oct 2019 - Article in J. Org. Chem. Published

Chris Nielsen's paper on the Hydroarylation of alkenes by protonation/Friedel-Crafts trapping is published.
Christian D.-T. Nielsen, Andrew J. P. White, David Sale, Jordi Burés and Alan Christopher Spivey, 'Hydroarylation of alkenes by protonation/Friedel-Crafts trapping – HFIP-mediated access to per-aryl quaternary stereocentres' J. Org. Chem. 2019, 84, 4965-14973, DOI: 10.1021/acs.joc.9b02393
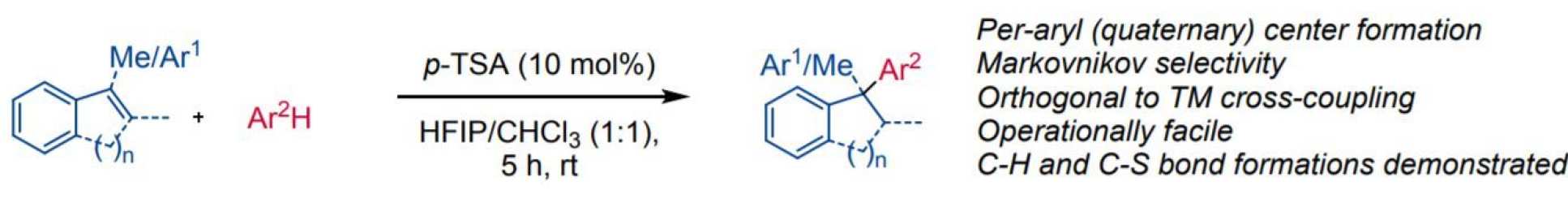
Upon treatment with a combination of HFIP and an organic sulfonic acid, alkenes behave as Brønsted bases and protonate to give carbocations which can be trapped by electron-rich arenes. The reaction constitutes a Friedel-Crafts (FC) hydroarylation which proceeds with Markovnikov selectivity and is orthogonal to traditional metal-catalyzed processes. Intermolecular transfer hydrogenation and hydrothiolation under analogous conditions are also demonstrated.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Professor Alan C Spivey
Department of Chemistry