A caged E3 ligase ligand for PROTAC-mediated protein degradation
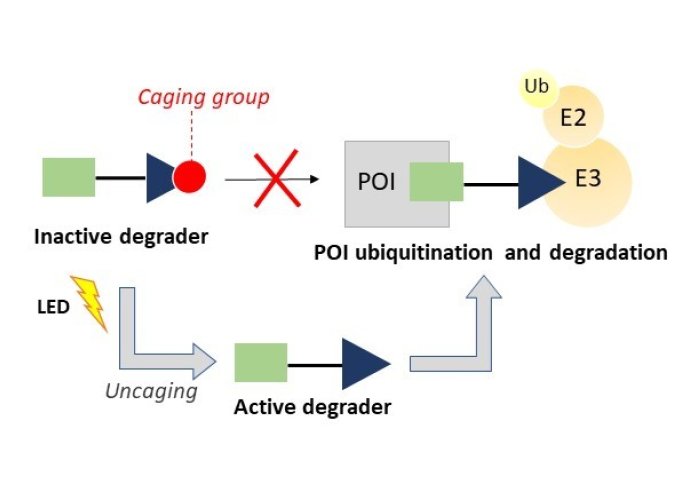
Photocaged PROTACs allow for the controlled release of the PROTAC
Congratulations to current group members Cyrille Koundé and Dr Maria Shchepinova, and former group member Dr Charlie Saunders, on their recent publication to Chem. Commun.
The research was conducted in collaboration with Cellzome GmbH and GlaxoSmithKline and aimed to achieve greater spatiotemporal control of PROTAC-induced protein degradation using light.
Proteolysis Targeting Chimeras (PROTACs) are powerful tools used to induce protein degradation. PROTACs are heterobifunctional molecules which consist of a ligand, aimed to interact with the protein of interest, and an E3 ligase recruiting motif, via a linker. PROTACs promote intracellular polyubiquitination and subsequent proteasome-dependent degradation of the targeted protein in a unique post-translational mode of action (complementing traditional gene and siRNA protein silencing strategies).
Until very recently, light-mediated small molecule regulation of mammalian proteins had not been studied extensively. Light-mediated PROTACs (or photocaged-PROTACs) offer an ingenious, yet simple, mechanism to control the release of PROTACs at the desired time or location. The results of the study demonstrated, upon photocleavage (uncaging), that the PROTACs were able to reduce cell proliferation just as efficiently as a PROTAC without the photocage design. The study supports the possibility of a wide application for varieties of future photocaged-PROTACs.
We would like to thank both the EPSRC and BBSRC for funding this work.
Well done to all those who were involved in the research!
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Ravi Singh
Department of Chemistry
Edward Bartlett
Department of Chemistry