A potential new route to future nerve regeneration technologies
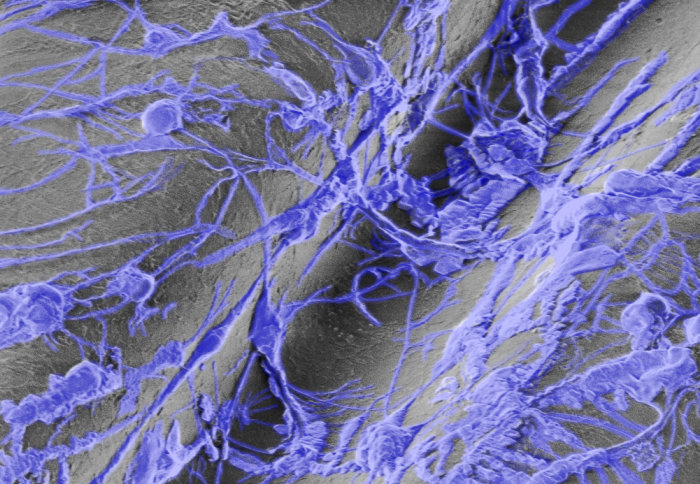
Hamlyn Centre researchers propose a possible new route to next generation nerve regeneration, by combining 3D printing, nanoscience and stem cells.
Trauma to peripheral nerves can lead to loss of innervation, loss of sensory and motor functions and even lifelong disability.
The peripheral neural system has a limited intrinsic ability to regenerate and regrow axons, with spontaneous recovery possible for small-gap injuries. However, if the gap is large, a form of bridging is required between the two nerve stumps to aid regeneration.

There are some major limitations to the common techniques employed to achieve regeneration of long sections of damaged nerves, such as direct suturing or autologous grafting. These limitations range from the need for post-surgery immune suppression to the limit of suitable nervous tissue that can be grafted from other parts of the body.
Thus, solutions involving biohybrid implants, where neural stem cells can be grown in vitro on active scaffolds before implantation, have attracted significant attention.
A novel bio-hybrid approach to induced neural stem cell differentiation on a drawn fibre scaffold
The Hamlyn Centre research team proposed a novel bio-hybrid approach to induced neural stem cell differentiation on a drawn fibre scaffold, aiming to aid the process of peripheral nerve regeneration.

Our researchers combined additive manufacturing, with nanoscience and neural stem cells to establish the feasibility of this potential new paradigm in engineering neural cell scaffolds.
Firstly, a polycarbonate fibre scaffold, fabricated by thermal drawing of a carefully designed and 3D printed preform was functionalised with poly-L-ornithine (PLO) and double-walled carbon nanotubes (DWCNTs). Then stem cells were cultured on the surface of the fibre cell scaffold and the effect of DWCNTs on the differentiation of neural stem cells into neural cells was investigated.
Using such an approach, the chemical and physical environment of cells can be tailored in order to control their differentiation and growth behaviours, which is crucial for bio-hybrid cell scaffolds for nerve regeneration.
Through this work, our researchers illuminate the potential use of this novel bio-hybrid approach for the realisation of future nerve regenerative implants. Furthermore, this work expands the toolbox of the thermally drawn fibre technique into the area of neural regeneration.
Indeed, this work is just the beginning on this potential new nerve regeneration approach. Whilst not investigated in their initial work, our researchers designed lumens into the cell scaffold.
These lumens allow for future investigation of the effect of delivery of electrical, chemical, optical and biochemical stimuli, and investigation into their synergy together with surface engineering, for the goal of a new paradigm in nerve regeneration.

This research was supported by EPSRC Programme Grant “Micro-robotics for Surgery (EP/P012779/1)” and was published in Biomedical Materials (Meysam Keshavarz, Dominic James Wales, Florent Seichepine, Mohamed E. M. K. Abdelaziz, Panagiotis Kassanos, Quan Li, Burak Temelkuran, Hongxing Shen and Guang-Zhong Yang, "Induced neural stem cell differentiation on a drawn fiber scaffold—toward peripheral nerve regeneration").
Images reproduced under the terms of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/) from Keshavarz et al., Biomedical Materials, 2020, 10.1088/1748-605X/ab8d12 © 2020 The Author(s). Published by IOP Publishing Ltd.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Erh-Ya (Asa) Tsui
Enterprise
Dominic Wales
Department of Electrical and Electronic Engineering