Peptide probes for Plasmodium falciparum MTIP
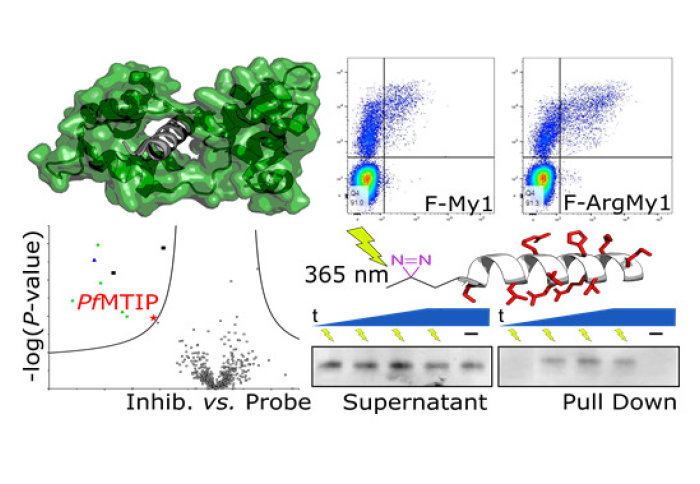
Peptide-based probes demonstrate low nanomolar binding affinity and enhanced cell penetration
Congratulations to former group member Dr Charlie Saunders on his recent publication in ACS Chemical Biology.
The collaborative research conducted by Dr Saunders, alongside Dr Ernesto Cota and Professor Jake Baum from the Department of Life Sciences, aimed to design and develop peptide-based probes to explore the druggability of the malaria-causing parasite Plasmodium falciparum.
Malaria remains a prevalent tropical disease with many front-line treatments failing against P. falciparum. This highlights an urgent need for new therapeutic agents to combat parasitic resistance. The Plasmodium glideosome complex contains several key protein-protein interactions which are thought to be essential for efficient invasion of the host red blood cell. At its core is a myosin motor (Myosin A) which provides most of the force required for parasite invasion.
The design of peptide-based probes for the anchor point of Myosin A, the P. falciparum Myosin A tail interacting protein (MTIP), is described in the publication. These probes combined low nanomolar affinity with significantly enhanced cell penetration. Through a combination of Western blot and chemical proteomics, these probes demonstrated competitive target engagement with native P. falciparum MTIP. The results of this study hope to provide new insights into the druggability of the MTIP-Myosin A interaction and a basis for the design of future inhibitors.
Dr Saunders is currently working for Professor Richard Houlston at the ICR. Here he is using bioinformatics to investigate the genetic and environmental risk factors for a wide range of cancer types.
This research was supported by the Institute for Chemical Biology, Wellcome, and EPSRC Centre for Doctoral Training.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Ravi Singh
Department of Chemistry
Edward Bartlett
Department of Chemistry