An overview of activity-based protein profiling and recent advances
A review of the last two years of advances in activity-based proteomics approaches
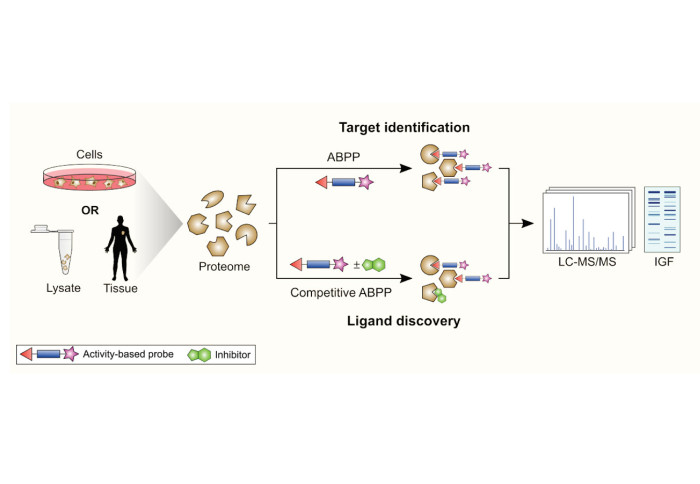
PhD student Henry Benns, joint member of Tate and Child groups, and Tate group alumnus Ceire Wincott reviewed activity-based protein profiling (ABPP), which was accepted for publication in Current Opinion in Chemical Biology. The study assessed the rise of class specific probes, including those that target both “writers” and “erasers” of protein modifications, processes that are frequently linked to human diseases. Also considered were several different approaches to the members of the protein degradation system, known as the ubiquitination pathway. Some probes make use of ubiquitin moieties, whilst others take a small molecule approach to improve cell-permeability issues.
The review also covered broad-spectrum reactivity-based probes. These types of probes target highly reactive amino acids and seek to identify druggable targets sites on proteins. For example, a proteomic study of cysteine hyperreactivity predicted which cysteine residues were likely to have functionality in the cell. This technique has been recently expanded to study the interactions of small molecules with cysteine residues by competitive screening. Now, several other amino acids have been interrogated by reactivity-based probes to produce new targets for inhibition and measurement.
Finally, the emergence of photoactivatable probes was investigated, both photoreactive and photocaged. Photoactivable probes can be used to target molecules where covalent binding may not be possible. These probes are active by application of UV light to either create a highly reactive radical, or to release a cage around the electrophilic warhead. The review concluded with a look to the future of ABPP and the targets that have been set for human proteomics research.
The research was funded by the BBSRC.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry