Understanding new gen materials for low cost hydrogen from sunlight and water
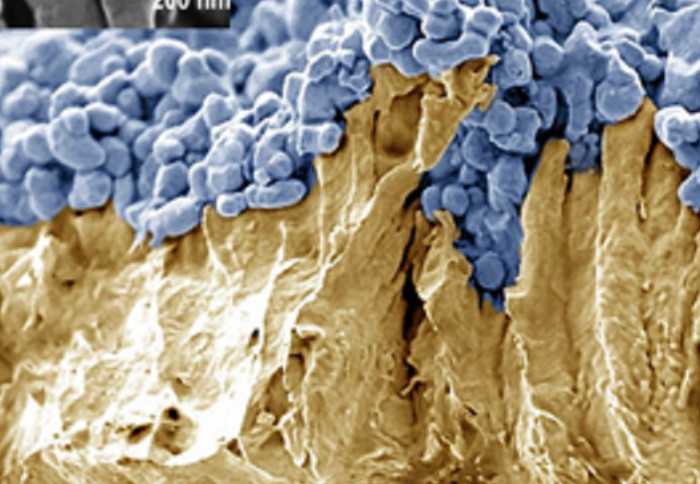
An electron microscope image of the photocatalyst sheets used in the study. La,Rh:SrTiO3 particles (blue) on a support metal (gold)
Understanding a new generation of materials for low cost hydrogen production from sunlight and water
A study just published in Nature Materials led by researchers at Imperial College London reports new insights into the working principle of a promising new device for driving low-cost production of green hydrogen.
Storing solar energy on a large scale is a key challenge which must be met in order to transition to a cleaner future. Plants have long specialised on converting the energy contained in sunlight to make sugars, which fuel all life on earth. By replicating key processes that occur in natural photosynthesis, carbon neutral fuels such as hydrogen can be produced in artificial systems using sunlight to help displace fossil fuels in sectors such as aviation and shipping, where technology power demands are too high for batteries to be economical.
Recently, researchers at the University of Tokyo have attracted global interest by developing a ‘photocatalyst sheet’ device that produces hydrogen with solar-to-fuel conversion efficiencies that match or even exceed natural photosynthesis. Crucially, photocatalyst sheets use low cost nanoparticles as their main component and can be fabricated in a single step. This means that photocatalyst sheet devices can in principle be mass produced at extremely low cost.
A key breakthrough supporting higher efficiencies in these sheets has been the development of novel photocatalyst nanoparticles of SrTiO3 which contain small amounts of Rhodium and Lanthanum (La,Rh:SrTiO3) SrTiO3 has been studied as a photocatalyst for the past 50 years, but because of its transparency to visible light, its solar energy conversion efficiency is intrinsically limited by the relatively small fraction of UV light in the solar spectrum. However, small amounts of impurities can induce visible light absorption. For example, the addition of small amounts of Rh into the SrTiO3 host lattice transforms the initially white powder, which only absorbs UV light, to a coloured powder which absorbs also in the visible.
Now, a team at Imperial College London has focused on explaining these colour changes and identifying the key factors which enable La,Rh:SrTiO3 in the photocatalyst sheets to operate efficiently where in the past many similar devices have failed. Crucially, the team found that incorporating Rh alone is not sufficient to create an efficient photocatalyst, which not only has to absorb a bigger chunk of sunlight but also needs to create high-energy electrons and store them until they can participate in chemical reactions (such as the splitting of water into hydrogen and oxygen). By combining spectroscopy, electrochemistry and simulations the team and their collaborators found that the success of the photocatalyst sheet device depends on the interplay of the added impurities (Rh with La) in the SrTiO3 host crystal lattice. The team found that Rh impurities can modify the energy landscape of the host crystal lattice by changing the energy levels of electrons in the material. Remarkably, they found that changing the oxidation state of Rh (Rh4+ vs. Rh3+) changes the number of levels, transforming purple Rh4+ ions in SrTiO3 into a yellow Rh3+ ions. Remarkably only the yellow material makes a good photocatalyst as it is able to store high-energy electrons for long enough till they can engage in fuel synthesis. Adding La is therefore crucial to prevent the formation of Rh4+, which contributes an energy level which consumes all of the high-energy electrons generated by sunlight before fuel can be produced.
By applying these and other lessons learned from this work to new photocatalyst particle designs, the researchers hope that more efficient devices which can harvest even larger fractions of the solar spectrum can be produced by the research community in the near future. It is hoped that this new generation of photocatalyst sheet devices will unlock large scale production of green hydrogen at reasonable cost.

The full article is available in Nature Materials: https://doi.org/10.1038/s41563-020-00868-2
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Benjamin Moss
Department of Materials
Lisa Bushby
Department of Physics