N-myristoyltransferase inhibition selectively kills MYC-deregulated cancer cells
Congratulations to joint first authors Gregor Lueg, Monica Faronato and Andrii Gorelik on their recent preprint
The preprint, available on biorxiv, details how N-myristoyltransferase inhibition selectively kills MYC-deregulated cancer cells.
Human N-myristoyltransferases (NMTs) are enzymes that catalyze N-terminal protein myristoylation, a modification regulating many cellular processes including membrane trafficking. NMT is considered a promising target in cancer and has been widely studied in the group for many years but a mechanistic rationale for targeted therapy has been elusive.
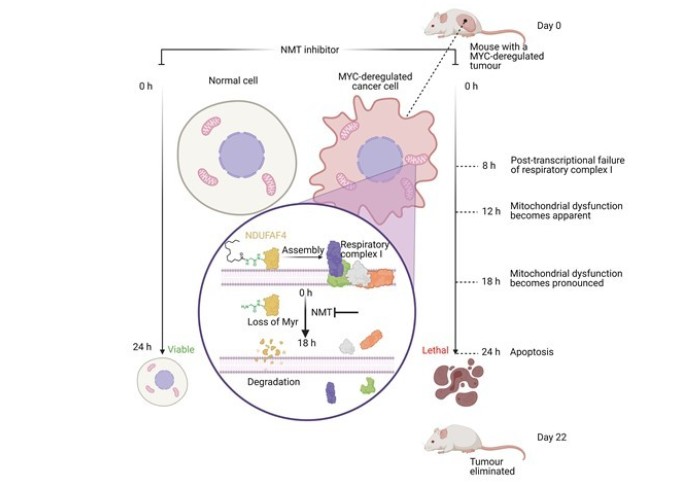
In this work, hundreds of cancer cell lines were screened against panel of potent and selective N-myristoyltransferase inhibitors (NMTi) and a strong correlation was found between increased NMTi sensitivity and MYC deregulation. MYC is an oncogene that is deregulated in over half of cancers but has previously proven difficult to target. Systems-level analyses revealed that NMTi is synthetic lethal with deregulated MYC and death of high MYC cancer cells treated with NMTi began after 24 hours.
SILAC proteomics and RNA-sequencing led to the discovery that NMTi cause mitochondrial complex I defects in high-MYC cancer cells followed by mitochondrial dysfunction. Mechanistically, NMTi-induced mitochondrial failure was found to be concurrent with loss of myristoylation on respiratory complex I assembly factor NDUFAF4 with subsequent proteasomal degradation.
The paper concludes by showing that an orally bioavailable NMTi eliminates MYC-deregulated tumours in vivo without overt toxicity, circumventing previous difficulties in targeting MYC.
This work was a large collaborative effort involving work from six members of the Tate group, co-supervised by Dinis Calado at The Francis Crick Institute with contributions from the University of Liverpool's Institue of Integrative Biology, Myricx Pharma, the Wellcome Sanger Institute, Kings College London, EPO Berlin and MDC, Berlin. Congratulations to all involved!
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Jennie Hutton
Department of Chemistry