How Imperial’s clinical researchers rose to the challenge of vaccine trials
by Ellyw Evans
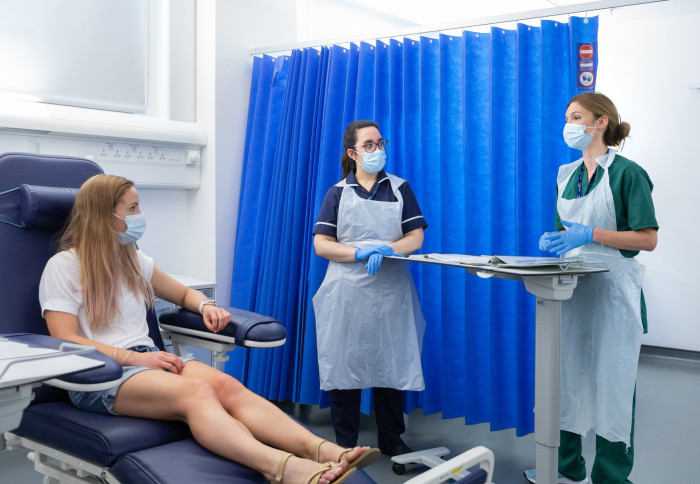
We speak to Imperial's Clinical Research Facility on how they adapted their work during the pandemic to deliver several COVID-19 vaccine trials.
On International Clinical Trials Day, we recognise the work of the National Institute for Health Research (NIHR) Imperial Clinical Research Facility (CRF) and their involvement in delivering COVID-19 vaccine trials.
The CRF is a partnership between Imperial College Healthcare NHS Trust and Imperial College London. The Facility offers a dedicated and purpose-built space in Hammersmith Hospital where researchers can deliver early phase clinical trials.
In response to the pandemic, the CRF halted their 140 existing studies across a variety of therapy areas to focus exclusively on delivering trials for candidate COVID-19 vaccines. In April 2020, the CRF became one of the first recruiting centres that the Oxford Vaccine Group approached to help deliver their study, COV001.
At its peak, the CRF was running three vaccine trials simultaneously at Hammersmith Hospital – Oxford’s COV001 and COV002, and Imperial’s COVAC1 – as well as the Janssen Ensemble-2 trial at Charing Cross Hospital.
We spoke to some of the team members involved in running the Facility and trials who share their experience of how it all unfolded, what they’ve learned, and their hopes for future clinical research.
- Dr Karen Mosley, General Manager
- Dr David Owen, Head of Clinical Studies
- Dr Katrina Pollock, Principal Investigator of vaccine studies
How were you able to switch gears so quickly to deliver the vaccine trials?
David: There were two key components that allowed us to move so fast; moving all staff onto this project and closing all other studies except for a few never-stop studies such as oncology. We received clear direction from the College and Trust that we should stop everything to focus on vaccine trials. Since then, all the studies that were put on hold have now restarted.
The response we received from our community was enormous. From professors to newly qualified doctors, many people donated their time. There is no doubt that their help massively increased our capacity
Karen: We were also lucky to receive support from non-clinical staff, including administrative staff who helped with the data and the Estates team who secured parking for trial participants. There was an amazingly cooperative atmosphere.

What challenges did you face along the way?
Katrina: One of the greatest challenges was ensuring that we delivered our trials safely against the backdrop of the pandemic. Suddenly we had much more staff in the unit and a massively increased footfall. Training all our staff on PPE and adhering to social distancing was a big piece of work, but I'm proud to say that while we have been working during the pandemic we have had no transmission in the unit.
David: Space has also been a challenge as this centre was not set up to deal with up to 60 participants a day so the logistics of making it all work were fairly complex. Having a separate space from the CRF where we could assess participants that develop COVID-19 symptoms was important, so we took over what was the nursery until a few years ago for this purpose.
We were also lucky to be able to take over the Imperial Research Hub at Charing Cross Hospital for additional space for symptomatic volunteers – it's actually a pod in the car park! That's where we're running the Janssen vaccine study.
How do you look back on the experience of running the trials now?
Katrina: It was an interesting time, a little exciting, but also very exhausting for all of us. But we kept going and we are pleased its yielded rewards.
Karen: I think it's just incredible that the vaccines worked because we've done a lot of vaccines trials over the years that haven't worked. The success of the vaccines in the pandemic is a bit of a miracle.
Do you think the increased public attention on clinical trials is positive?
David: As a clinical pharmacologist, I massively enjoyed the fact that there were several months when the question on everyone's lips was how the clinical trial is doing. It was great that it caught the national agenda so much, but I'm cynical that this will last.
Karen: The increased attention had a very positive impact on our trial recruitment – across the Oxford and Imperial studies, we had around 15,000 people apply which is great! There have also been many positive stories from trial participants on social media and on the news, and this will hopefully encourage people to volunteer for future studies. 
What have you learnt from this experience, and will it change the future way of working for the CRF?
Karen: This experience has shown us that we can be flexible and adapt very quickly when we have to. If you'd asked us the year before the pandemic if we could suddenly take on these studies and recruit 60 people a day, we would have laughed and walked away! But we can, and that's reassuring. Our ethics and regulatory bodies have also proved they can be flexible and quick to adapt.
In terms of our future way of working, we're looking to develop a hub and spoke model, where the CRF is the main early phase research facility across the Trust and College with links into all the other infrastructure out there. The more we can link, the more adaptable we can be in the future if we need to.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Ellyw Evans
Faculty of Medicine Centre


