A Novel Plasmonic Optical Fibre Approach for Bacterial Accumulation Behaviour
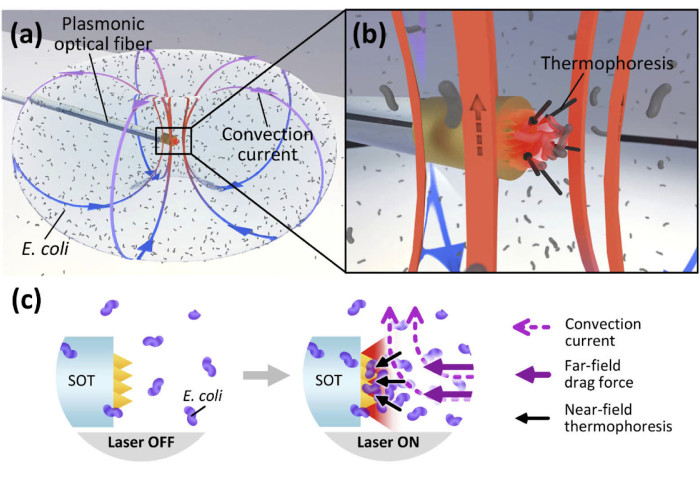
Our Hamlyn researchers proposed a plasmonic optical fibre approach for bacterial accumulation behaviour, aiming to aid bacteria-based drug delivery.
Bacteria can potentially be employed as natural micro-robots for targeted drug delivery owing to their autonomous behaviour in response to the surrounding environment. However, this autonomous motion of bacteria is slow and is limited in transport length when considering its targets (e.g. tissues, organs) due to the difference in size between bacteria (a few microns) and tissues and organs (up to several centimetres).
External energy sources—such as magnetic fields, acoustic waves, and optical tweezers—therefore have been used to enhance guidance and transport of bacteria (and of nano/micro-particles and micro-robots). It is evidence-proved that robust and non-invasive actuation, transport and control of bacteria (and of other untethered micro-objects and micro-robots) may provide numerous opportunities for clinical applications.
In the field of drug delivery, controlling bacterial ‘swarms’ has been proposed as a method for targeting bacteria to specific regions in order to deliver payloads of drugs. Nevertheless, numerous limitations still remain in magnetic micro-manipulation of bacterial swarms (it is limited to specific bacterial species; magnetic functionalisation can be required; and strong magnetic fields are required), as well as in conventional optical tweezers approaches (they may not be suitable for generating swarming behaviour in micro-objects).
Plasmonic optical fiber for bacteria manipulation
To overcome the obstacles mentioned above, our researchers at the Hamlyn Centre proposed a novel plasmonic optical fibre approach to characterise and visualise the bacterial accumulation behaviour under plasmo-thermal trapping.
Our research team demonstrated a plasmo-thermal high-density bacterial accumulation effect by using a miniature plasmonic optical fibre (plasmonic microstructures on the tips of optical fibres that is fabricated via two-photon polymerisation and gold sputtering), which potentially can be applied in future in-vivo applications such as bacteria-based drug delivery.

The combined action of far-field convection and a near-field trapping force (referred to as thermophoresis)—induced by highly localised plasmonic heating—enabled the large-area accumulation of Escherichia coli. The estimated thermophoretic trapping force agreed with previous reports.


Moreover, our researchers applied speckle imaging analysis to map the in-plane bacterial velocities over large areas for demonstrating the uniformity of the accumulation effect.
It is worth to mention that this is the first time that spatial mapping of bacterial velocities has been achieved in this setting. Thus, this analysis technique provides opportunities to better understand this phenomenon and to drive it towards in-vivo applications.
Last but not least, as this novel approach uses the white light images taken using a general optical microscope (unlike other approaches that require laser illumination), it offers greater versatility and potential for wider clinical applications.
This research was supported by EPSRC Programme Grant, “Micro-robotics for Surgery (EP/P012779/1)” and NIHR Imperial Biomedical Research Centre (1215-20013) (Jang Ah Kim, Alexander J. Thompson and Eric Yeatman, "Plasmonic optical fiber for bacteria manipulation—characterization and visualization of accumulation behavior under plasmo-thermal trapping", Biomedical Optics Express, 12 (7), 3917-3933, June 2021).
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Jang Ah Kim
Department of Mechanical Engineering
Erh-Ya (Asa) Tsui
Enterprise