Blood clots in severe COVID cases may be related to abnormal antibody response
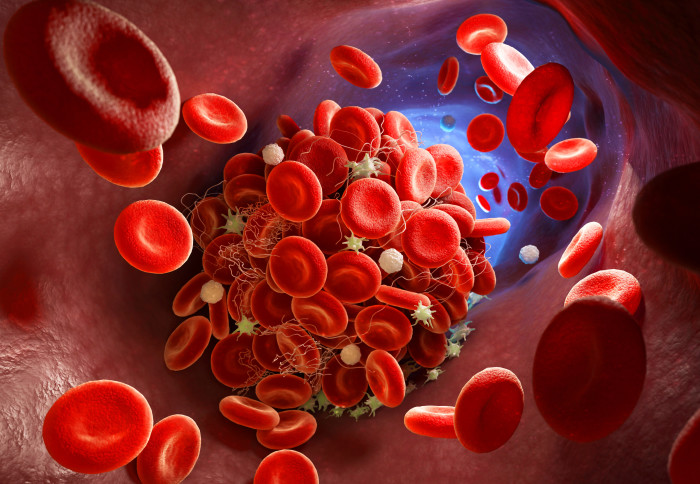
Inflammation and blood clotting seen in severe cases of COVID-19 may be caused by antibodies activating unnecessary platelet activity in the lungs.
This is the key finding of a study by researchers at the University of Reading and Imperial College London, published in the journal Blood.
Platelets are small cells found in the blood that form clots to stop or prevent bleeding. However, when platelets malfunction, this can lead to serious health concerns such as strokes and heart attacks.
This lab-based study of human cells provides key evidence to support the scientific basis for the MATIS trial led by Imperial College London and Imperial College Healthcare NHS Trust, which is already testing two drugs (fostamatinib or ruxolitinib) to see whether they will reduce serious clotting for hospitalised COVID-19 patients.
While there are yet to be any results reported from MATIS, the trial team and researchers behind the current study will continue to work closely together as the clinical trial develops.
Immune overreaction
The study took antibodies produced to fight the coronavirus’s spike protein from people with severe COVID-19 infection and cloned them in a lab to study. The team found that the small sugars found on the surface of these antibodies were different to antibodies from healthy individuals. Furthermore, when these cloned antibodies were introduced in a lab to blood cells taken from healthy donors, there was an observed increase in platelet activity.
In the lab, the team also found that it was possible to reduce or stop platelets from responding in this way by introducing the active ingredient from a drug used to dampen the immune response in other diseases. The findings suggest that it may be possible for drugs that are currently used to treat immune system problems to reduce or stop the cells from producing an exaggerated platelet response.
Clotting clues
Professor Jon Gibbins, Director of the Institute for Cardiovascular and Metabolic Research at the University of Reading said: “Until now, we have only had assumptions about why platelets involved in clotting were being activated during COVID-19 infection.”
“One way to think of what is happens is that the immune response that is designed to protect you from the infection in some cases, particularly in severely ill patients, actually causes more damage. In this case, the antibodies that are produced to stop COVID-19 from spreading trigger infected cells to induce platelet activity which causes clotting even though there is no wound that needs healing.”
“We are particularly excited because our studies of platelets in the laboratory establish important mechanisms that explain how and why dangerous blood clots may occur in severely ill COVID-19 patients, and importantly, also provides clues as to how this may be prevented.”
Co-author Dr Nichola Cooper, Clinical Reader in Immunohaematology at Imperial and consultant haematologist at Imperial College Healthcare NHS Trust, who also designed and leads the MATIS trial said: “Early on in the COVID-19 pandemic it was clear that the infection was causing an overwhelming immune response, including blood clotting, and that many of the more severe cases and deaths were related to this.”
“Having been involved in early research around blood clotting related to inflammation, it occurred to me that the drugs we already use for other disorders could be easily accessible treatments for COVID-19.”
“We are yet to see results from the MATIS trial so we do not yet know how these drugs will work in patients, but our hope is that we can both inhibit the inflammatory response and prevent severe disease and blood clots. It is exciting to see our collaboration with Reading backing our theory already and providing a solid scientific basis for clinical trials.”
‘Aberrant glycosylation of anti-SARS-CoV-2 IgG is a pro-thrombotic stimulus for platelets’ by Alexander P Bye et al. is published in Blood.
This article has been adapted from a press release by the University of Reading
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Ms Genevieve Timmins
Academic Services