Mar 2021 - Article Published in J. Org. Chem.

J Org Chem
Christian Neilsen's paper on the resolution of lactols exploiting a steroelectronic relay is published in J. Org. Chem.
Carla Alamillo-Ferrer, Christian D.-T. Nielsen, Andrea Salzano, Xavier Companyó, Riccado Di Sanza, Alan C. Spivey, Henty S. Rzepa, and Jordi Burés ’Understanding the Diastereopreference of Intermediates in Aminocatalysis: Application to the Chiral Resolution of Lactols’ J. Org. Chem. 2021, 86, 4326−4335. DOI: https://doi.org/10.1021/acs.joc.0c02998
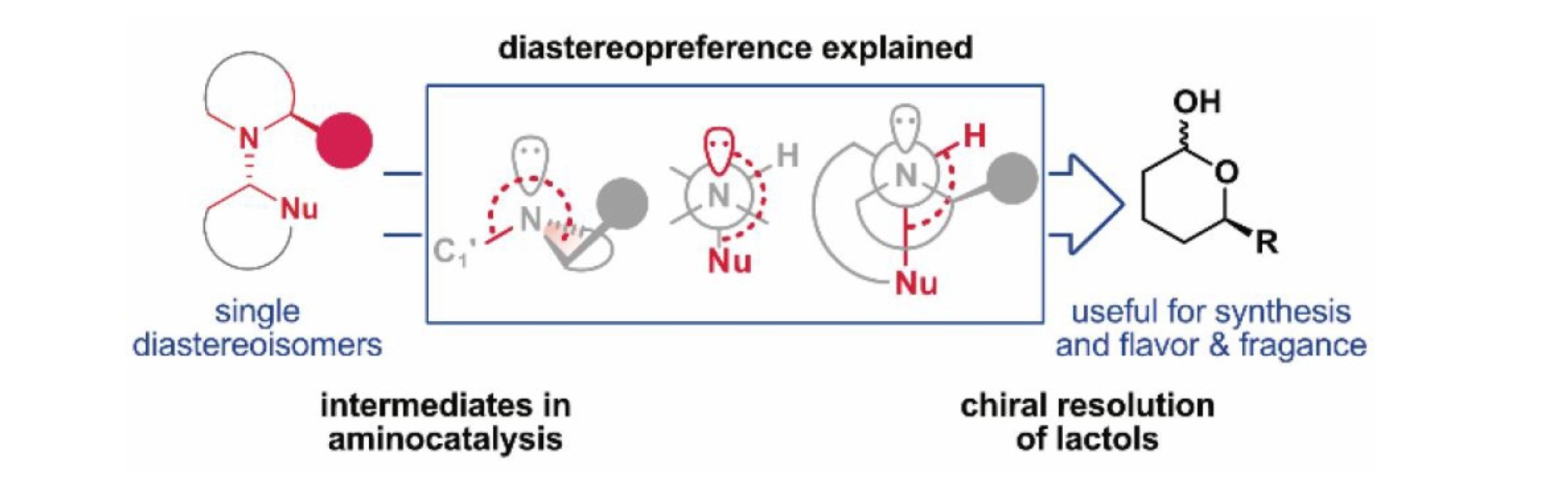
Downstream intermediates are crucial for the reactivity and selectivity of aminocatalytic reactions. We present an analysis of the stereopreference in aminocatalytic downstream intermediates, which reveals an inconspicuous mechanism of chiral recognition between the catalyst and the rest of the molecule. We delineate a stereoelectronic model to rationalize the mode of chiral transmission. We also exploit it for the resolution of chiral lactols relevant in organic synthesis as well as in the flavor and fragrance industries.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Professor Alan C Spivey
Department of Chemistry