Research reveals how ‘chaperone’ proteins deal with immune system overreactions
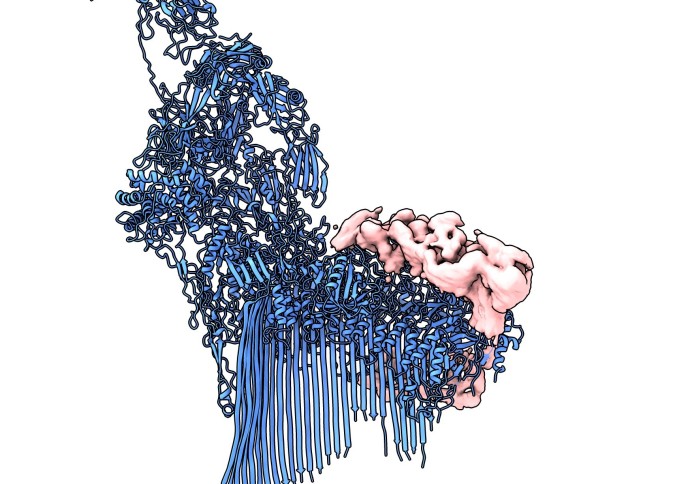
Chaperone protein (pink) and an immune attack complex (blue)
Scientists have discovered how special proteins in the blood prevent our immune system from harming our own cells with overproduced molecules.
The research, led by Imperial College London scientists and published today in Nature Communications, provides insights into how our body keeps the immune system in check. It could also point to how special chaperone proteins can prevent the accumulation of other harmful molecules, such as those associated with Alzheimer’s disease.
Seeing how these proteins stop MAC provides the first clues into how this branch of the immune system can be controlled Dr Doryen Bubeck
The team studied membrane attack complexes (MACs) – components of our immune system that punch minute holes in the membrane of invading bacteria. If enough holes are punched, the bacteria will pop and die.
The immune system therefore creates loads of MACs when an invader is detected, but not all of these reach their targets, meaning many end up in the bloodstream, where they could potentially damage the body’s own cells.
Scientists knew special chaperone proteins – called clusterin and vitronectin – helped to prevent these MACs from causing unwanted attacks, but didn’t know how.
Now, the team from Imperial and institutions in the Netherlands have managed to capture and investigate in unprecedented detail MAC precursor molecules bound to the chaperone proteins, revealing how the chaperones stop MACs becoming fully functional.
Capturing rogue molecules
Lead researcher Dr Doryen Bubeck, from the Department of Life Sciences at Imperial, said: “When a pathogen is detected, our immune system goes into overdrive to make MACs and not all of them reach their bacterial targets. Here we discovered how chaperones in the blood capture rogue molecules and prevent them from damaging human cells.
“Seeing how these proteins stop MAC provides the first clues into how this branch of the immune system can be controlled and shows us how these chaperones might capture other harmful proteins in the blood.”
From their detailed investigation, the team showed that clusterin attaches to a precursor version of MACs that is dissolved in the bloodstream. It then prevents the MAC from building up more of the components it needs to fully assemble and carry out its hole-punching attack.

It’s this prevention of build-up of further material that the researchers say could provide interesting insights into other material accumulations. For example, beta-amyloid fibres, if they are allowed to accumulate, can lead to the plaques that characterise Alzheimer’s disease.
First author Anaïs Menny, also from the Department of Life Sciences at Imperial, said: “If clusterin uses the same method to recognise and prevent beta-amyloid accumulation as it does for MACs, then we could get some really interesting insights into how the early precursors to Alzheimer’s disease arise.”
-
‘Structural basis of soluble membrane attack complex packaging for clearance’ by Anaïs Menny, Marie V. Lukassen, Emma C. Couves, Vojtech Franc, Albert J.R. Heck and Doryen Bubeck is published in Nature Communications.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division