Bioengineers find new way heart valves grow – and go wrong
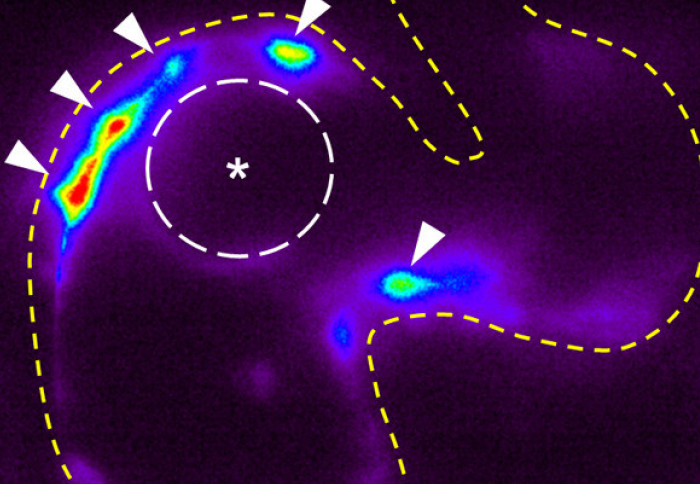
Imperial bioengineers have discovered a new mechanism driving the growth of heart valves in zebrafish embryos.
The findings, from a paper from National Institute of Health and Medical Research (Inserm) in France, University of Strasbourg, and Imperial College London, shed light on how heart valves grow and find their shapes in embryos. They could also help us understand why some valves don’t develop properly, potentially leading to new avenues for treatment.
We have discovered a pathway that’s crucial for heart valve development...our findings could help to inform the future prevention and treatment of diseases of the heart valves. Dr Julien Vermot Department of Bioengineering
In humans, the heart is divided into four chambers – two atria at the top, which receive blood, and two ventricles at the bottom, which pump blood back into the body. Blood pumps around the heart via a one-way system of four valves that prevent back-flow. As the heart contracts and relaxes, the valves open and shut, letting blood flow into the atria and out of the ventricles at alternate times.
Heart valves are constantly challenged by the mechanical forces generated by heartbeat, and diseases of the heart valves can mean that blood fails to circulate properly, potentially causing heart failure, stroke, and death. Some people are born with diseased heart valves - known as congenital heart valve defects - but exactly how valves grow in embryos is poorly understood.
The research team used zebrafish to identify the mechanosensitive processes at play during the development of the valves found between atria and ventricles, known as atrioventricular valves.

They found that alongside mechanisms that were already known to generate heart valve tissue, another mechanism works in parallel to determine the shape and function of the atrioventricular canal (AVC), from which the atrioventricular valves develop. Further studying the role of mechanical forces in congenital valve defects could eventually help understand how to prevent and treat them.
Co-author Dr Julien Vermot of Imperial’s Department of Bioengineering said: “Mechanical forces in embryos may determine the shape of many organs in the body. We have discovered a pathway that’s crucial for heart valve development, and our findings could help to inform the future prevention and treatment of diseases of the heart valves.”
Beating hearts
To carry out the study, the researchers studied the roles of calcium ions (Ca2+) and adenosine triphosphate (ATP), and established that these signaling molecules are activated by mechanical forces in the zebrafish heart. They found that these factors contribute to mechanisms in heart valve cell creation that are driven by the mechanical forces activated by the beating heart.
Dr Vermot added: “This work further unravels how mechanical forces can influence tissue remodeling in developing organs.”
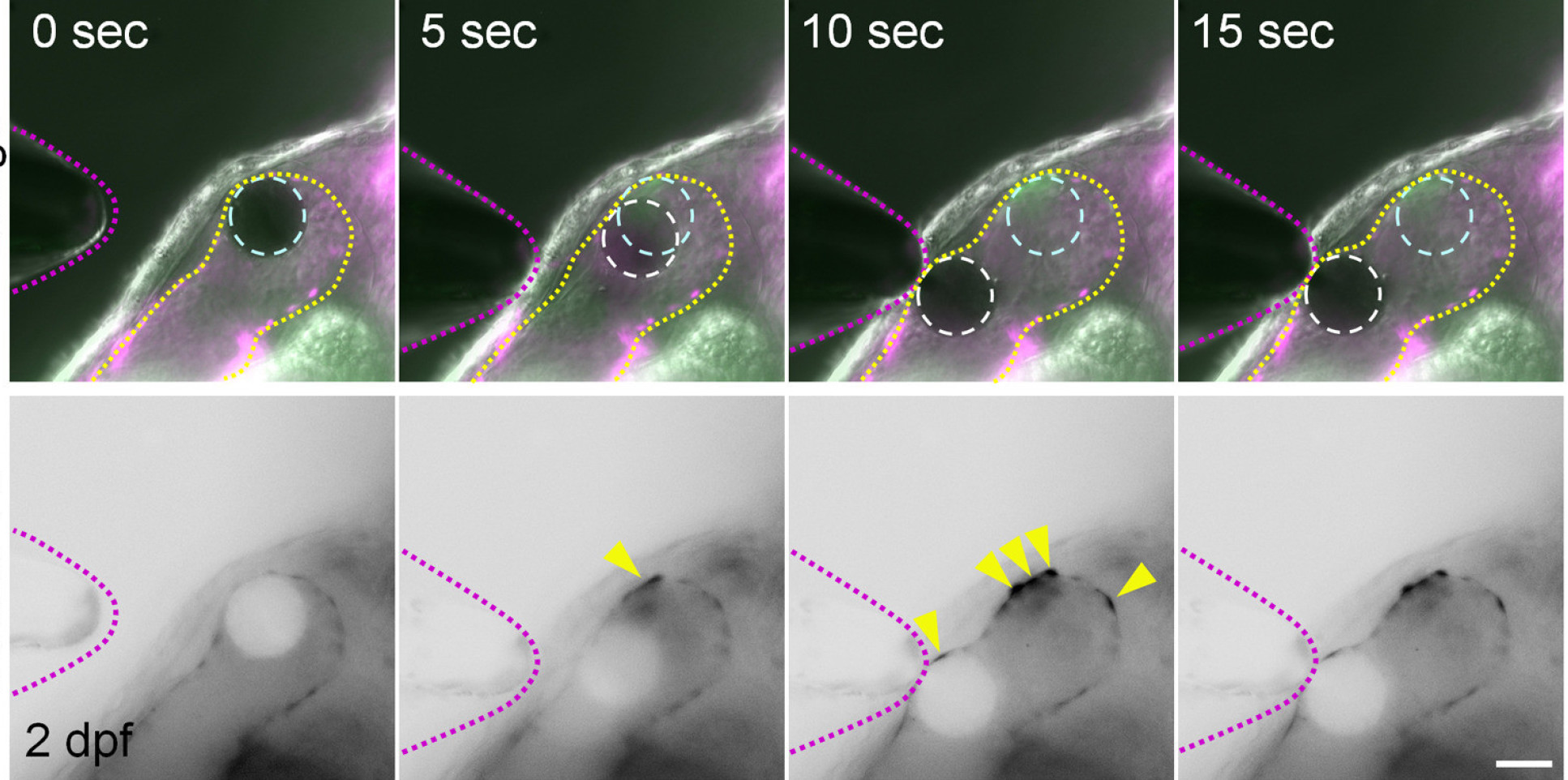
The researchers say their findings could help us better understand how mechanical forces affect cell differentiation and what their role might be in producing malformed valves. They could lead to drugs, treatments, and even be used to help growing replacement valve for patients with valve defects.
Next, the researchers will look at how the pathway interacts with others and how their findings might translate into tissue engineering and in other organisms like mice and humans.
“Bioelectric signaling controls cardiac valve position and cell fate in response to mechanical forces” by Hajime Fukui, Renee Wei-Yan Chow, Jing Xie, Yoke Yin Foo, Choon Hwai Yap, Nicolas Minc, Naoki Mochizuki, and Julien Vermot. Published 15 October 2021 in Science.
Images: Fukui et al.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Caroline Brogan
Communications Division