Identification of the first covalent ligands of the small GTPase Rab27A
A collaborative effort, led by the Tate group, paves the way for the structure-guided discovery of small molecules against Rab27A
Rab27A is a small GTPase that represents an extremely challenging target for drug discovery and has been shown to promote the growth and invasion of cancers (such as breast, lung, and pancreatic), by promoting the secretion of chemokines, metalloproteases, and exosomes. Metastasis is still a critically unmet clinical need and putative protein targets, such as Rab27A, are important, yet understudied, drug discovery targets within the field.
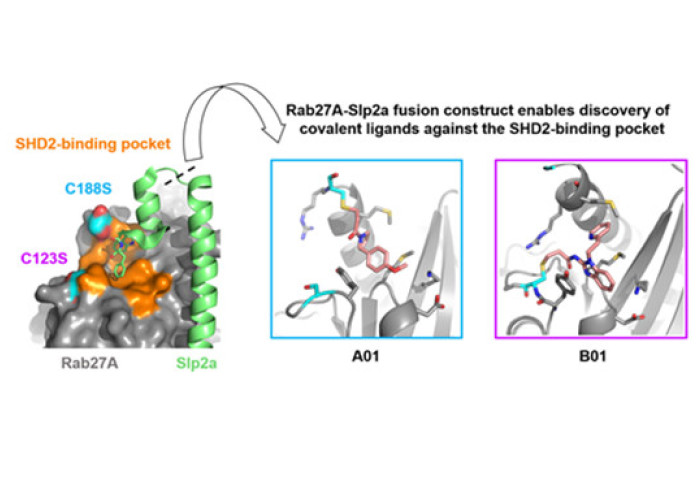
Exploiting protein engineering strategies, our RSC Medicinal Chemistry article showcases a novel construct of Rab27A, fused to a portion of the effector protein Slp2a, has enabled high-throughput X-ray crystallography of Rab27A for the first time. This biochemical tool is key for the study and development of binders that occupy a conserved pseudo-pocket on Rab27A. Such molecules would have the potential to be exploited to block important protein-protein interactions that are required for the activity of Rab27A. Two flanking cysteine residues found at this pocket have been successfully used as anchoring points for the discovery of covalent fragment binders, which could be used to optimize targeted covalent inhibitors of Rab27A.
The research highlights joined efforts from the Rab27A team in the Tate group, with the leadership of alumnus Dr Mostafa Jamshidiha (Structural Biology) and Dr Thomas Lanyon-Hogg (University of Oxford) and leverages the novel quantitative irreversible tethering (qIT) platform developed in collaboration with the group of Dr David Mann (Life Sciences). These results represent the tip of the iceberg in unravelling the drug discovery against the challenging GTPase Rab27A, which have been conducted alongside Prof. Jim Norman (Beatson Institute).
We are grateful to our funders to enable this exciting research. The work was funded by a Drug Discovery Committee award from Cancer Research UK, the Imperial Confidence in Concept (ICiC) scheme and further supported by two Horizon 2020 Marie Sk?odowska Curie actions (to Dr Elena De Vita and Dr Rita Petracca). The structural biology was conducted in collaboration between the Imperial College X-ray facility and the Diamond Light Source.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Ravi Singh
Department of Chemistry