Light-mediated multi-target protein degradation using photoswitching
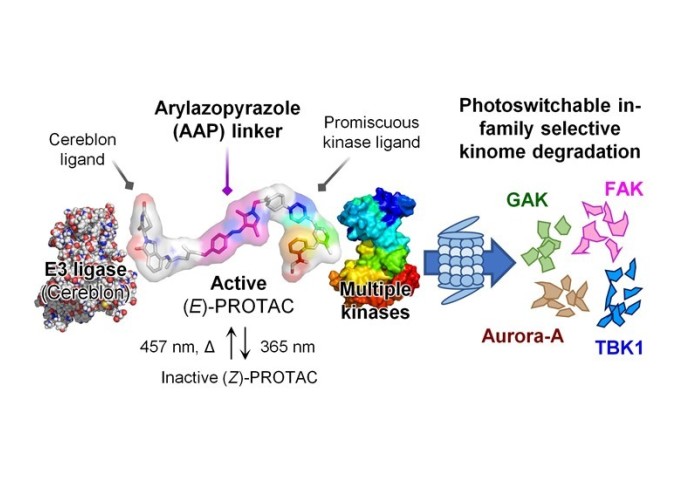
A collaborative project, led by Tate group, has combined PROTAC technology with light-activatable control to achieve protein degradation
PROteolysis-TArgeting Chimeras (PROTACs) are molecules which are able to bind to a protein of interest (POI) and lead to its degradation. By linking an E3 ligase ligand and a POI binder, PROTACs allow the formation of an E3 ligase/PROTAC/POI ternary complex which drives proximity-induced POI ubiquitination and subsequent degradation.
While many oncogenic protein targets have been successfully degraded by PROTACs, thereby showing their potential as a novel therapeutic modality for drug discovery, spatiotemporal control of protein degradation with light remains less explored, with only a few examples to date.
Our publication in Chem. Commun. reports a new class of photoswitchable PROTAC incorporating a novel arylazopyrazole photoswitch (AP-PROTACs) featuring differentiated photoswitching properties, and the first example of non-azobenzene PHOTAC technology. With a bromodomain (BRD) ligand (AP-PROTAC-1) or a multi-kinase inhibitor (AP-PROTAC-2) as the PROTAC warhead, light-switchable degradation of multiple proteins was achieved. In particular, it demonstrates the first example of light-tuneable degradation of multiple specific protein kinases using a broad-spectrum kinase inhibitor as the POI ligand.
A huge thanks to Prof. Matt Fuchter, Dr Dima Kozakov, GSK, and everyone in their teams for the valuable input and funding for this research project.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Ravi Singh
Department of Chemistry